Nrf2 and NF-κB and Their Concerted Modulation in Cancer Pathogenesis and Progression
- PMID: 24281078
- PMCID: PMC3835087
- DOI: 10.3390/cancers2020483
Nrf2 and NF-κB and Their Concerted Modulation in Cancer Pathogenesis and Progression
Abstract
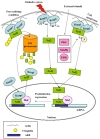
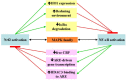
Reactive oxygen species, produced by oxidative stress, are implicated in the initiation, promotion, and malignant conversion of carcinogenesis through activation/suppression of redox-sensitive transcription factors. NF-E2-related factor 2 (Nrf2) encodes for antioxidant and general cytoprotection genes, while NF-κB regulates the expression of pro-inflammatory genes. A variety of anti-inflammatory or anti-carcinogenic phyto-chemicals suppress NF-κB signalling and activate the Nrf2-ARE pathway. In this review we consider the role of Nrf2 and NF-κB in cancer pathogenesis and progression, focusing on their concerted modulation and potential cross-talk.
Figures

References
-
- Nyaga S.G., Jaruga P., Lohani A., Dizdaroglu M., Evans M.K. Accumulation of oxidatively induced DNA damage in human breast cancer cell lines following treatment with hydrogen peroxide. Cell Cycle. 2007;6:1472–1478. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

