The nrf1 and nrf2 balance in oxidative stress regulation and androgen signaling in prostate cancer cells
- PMID: 24281119
- PMCID: PMC3835133
- DOI: 10.3390/cancers2021354
The nrf1 and nrf2 balance in oxidative stress regulation and androgen signaling in prostate cancer cells
Abstract
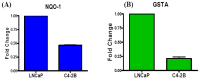
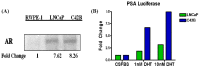
Reactive oxygen species (ROS) signaling has recently sparked a surge of interest as being the molecular underpinning for cancer cell survival, but the precise mechanisms involved have not been completely elucidated. This review covers the possible roles of two ROS-induced transcription factors, Nrf1 and Nrf2, and the antioxidant proteins peroxiredoxin-1 (Prx-1) and Thioredoxin-1 (Txn-1) in modulating AR expression and signaling in aggressive prostate cancer (PCa) cells. In androgen independent (AI) C4-2B cells, in comparison to the parental androgen dependent (AD) LNCaP cells, we present evidence of high Nrf1 and Prx-1 expression and low Nrf2 expression in these aggressive PCa cells. Furthermore, in DHT treated C4-2B cells, increased expression of the p65 (active) isoform of Nrf1 correlated with enhanced AR transactivation. Our findings implicate a crucial balance of Nrf1 and Nrf2 signaling in regulating AR activity in AI-PCa cells. Here we will discuss how understanding the mechanisms by which oxidative stress may affect AR signaling may aid in developing novel therapies for AI-PCa.
Figures


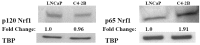


Similar articles
-
Oxidative stress and redox signaling in CRPC progression: therapeutic potential of clinically-tested Nrf2-activators.Cancer Drug Resist. 2021 Mar 19;4(1):96-124. doi: 10.20517/cdr.2020.71. eCollection 2021. Cancer Drug Resist. 2021. PMID: 35582006 Free PMC article. Review.
-
Nrf1 and Nrf2 transcription factors regulate androgen receptor transactivation in prostate cancer cells.PLoS One. 2014 Jan 22;9(1):e87204. doi: 10.1371/journal.pone.0087204. eCollection 2014. PLoS One. 2014. PMID: 24466341 Free PMC article.
-
RELA is sufficient to mediate interleukin-1 repression of androgen receptor expression and activity in an LNCaP disease progression model.Prostate. 2020 Feb;80(2):133-145. doi: 10.1002/pros.23925. Epub 2019 Nov 15. Prostate. 2020. PMID: 31730277 Free PMC article.
-
Identification of novel androgen receptor target genes in prostate cancer.Mol Cancer. 2007 Jun 6;6:39. doi: 10.1186/1476-4598-6-39. Mol Cancer. 2007. PMID: 17553165 Free PMC article.
-
Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer.Free Radic Biol Med. 2011 Oct 1;51(7):1320-8. doi: 10.1016/j.freeradbiomed.2011.07.011. Epub 2011 Jul 23. Free Radic Biol Med. 2011. PMID: 21820046 Review.
Cited by
-
Targeting Crosstalk between Nrf-2, NF-κB and Androgen Receptor Signaling in Prostate Cancer.Cancers (Basel). 2018 Sep 25;10(10):352. doi: 10.3390/cancers10100352. Cancers (Basel). 2018. PMID: 30257470 Free PMC article. Review.
-
Oxidative stress and redox signaling in CRPC progression: therapeutic potential of clinically-tested Nrf2-activators.Cancer Drug Resist. 2021 Mar 19;4(1):96-124. doi: 10.20517/cdr.2020.71. eCollection 2021. Cancer Drug Resist. 2021. PMID: 35582006 Free PMC article. Review.
-
Bardoxolone-Methyl (CDDO-Me) Suppresses Androgen Receptor and Its Splice-Variant AR-V7 and Enhances Efficacy of Enzalutamide in Prostate Cancer Cells.Antioxidants (Basel). 2020 Jan 12;9(1):68. doi: 10.3390/antiox9010068. Antioxidants (Basel). 2020. PMID: 31940946 Free PMC article.
-
HSP90 inhibitor 17-DMAG exerts anticancer effects against gastric cancer cells principally by altering oxidant-antioxidant balance.Oncotarget. 2017 Apr 10;8(34):56473-56489. doi: 10.18632/oncotarget.17007. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915605 Free PMC article.
-
Thioredoxin system-mediated regulation of mutant Kras associated pancreatic neoplasia and cancer.Oncotarget. 2017 Oct 4;8(54):92667-92681. doi: 10.18632/oncotarget.21539. eCollection 2017 Nov 3. Oncotarget. 2017. PMID: 29190947 Free PMC article.
References
-
- Cancer Facts and Figures 2009. American Cancer Society; Atlanta, GA, USA: 2009.
-
- Thalmann G.N., Anezinis P.E., Chang S.M., Zhau H.E., Kim E.E., Hopwood V.L., Pathak S., von Eschenbach A.C., Chung L.W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

