Genetic complexity in a Drosophila model of diabetes-associated misfolded human proinsulin
- PMID: 24281154
- PMCID: PMC3914625
- DOI: 10.1534/genetics.113.157602
Genetic complexity in a Drosophila model of diabetes-associated misfolded human proinsulin
Erratum in
- Genetics. 2014 Dec;198(4):1773
Abstract
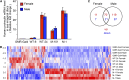
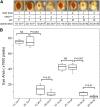
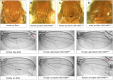
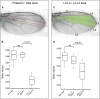
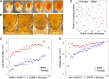
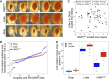
Drosophila melanogaster has been widely used as a model of human Mendelian disease, but its value in modeling complex disease has received little attention. Fly models of complex disease would enable high-resolution mapping of disease-modifying loci and the identification of novel targets for therapeutic intervention. Here, we describe a fly model of permanent neonatal diabetes mellitus and explore the complexity of this model. The approach involves the transgenic expression of a misfolded mutant of human preproinsulin, hINS(C96Y), which is a cause of permanent neonatal diabetes. When expressed in fly imaginal discs, hINS(C96Y) causes a reduction of adult structures, including the eye, wing, and notum. Eye imaginal discs exhibit defects in both the structure and the arrangement of ommatidia. In the wing, expression of hINS(C96Y) leads to ectopic expression of veins and mechano-sensory organs, indicating disruption of wild-type signaling processes regulating cell fates. These readily measurable "disease" phenotypes are sensitive to temperature, gene dose, and sex. Mutant (but not wild-type) proinsulin expression in the eye imaginal disc induces IRE1-mediated XBP1 alternative splicing, a signal for endoplasmic reticulum stress response activation, and produces global change in gene expression. Mutant hINS transgene tester strains, when crossed to stocks from the Drosophila Genetic Reference Panel, produce F1 adults with a continuous range of disease phenotypes and large broad-sense heritability. Surprisingly, the severity of mutant hINS-induced disease in the eye is not correlated with that in the notum in these crosses, nor with eye reduction phenotypes caused by the expression of two dominant eye mutants acting in two different eye development pathways, Drop (Dr) or Lobe (L), when crossed into the same genetic backgrounds. The tissue specificity of genetic variability for mutant hINS-induced disease has, therefore, its own distinct signature. The genetic dominance of disease-specific phenotypic variability in our model of misfolded human proinsulin makes this approach amenable to genome-wide association study in a simple F1 screen of natural variation.
Keywords: Drosophila; complex disease; diabetes; misfolded protein; mutant insulin.
Figures

References
-
- Bedell M. A., Jenkins N. A., Copeland N. G., 1997a Mouse models of human disease. Part I: techniques and resources for genetic analysis in mice. Genes Dev. 11: 1–10. - PubMed
-
- Bedell M. A., Largaespada D. A., Jenkins N. A., Copeland N. G., 1997b Mouse models of human disease. Part II: recent progress and future directions. Genes Dev. 11: 11–43. - PubMed
-
- Bell G. I., Swain W. F., Pictet R., Cordell B., Goodman H. M., et al. , 1979. Nucleotide sequence of a cDNA clone encoding human preproinsulin. Nature 282: 525–527. - PubMed
-
- Benjamini Y., Hochberg Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy. Statist. Soc. Ser. B 57: 289–300.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

