Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo
- PMID: 24281189
- PMCID: PMC4104160
- DOI: 10.1161/CIRCRESAHA.114.302113
Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo
Abstract
Rationale: The kynurenine (Kyn) pathway is the major route for tryptophan (Trp) metabolism in mammals. The Trp-Kyn pathway is reported to regulate several fundamental biological processes, including cell death.
Objective: The aim of this study was to elucidate the contributions and molecular mechanism of Trp-Kyn pathway to endothelial cell death.
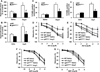
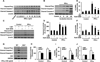
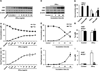
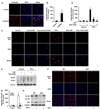
Methods and results: Endogenous reactive oxygen species, endothelial cell apoptosis, and endothelium-dependent and endothelium-independent vasorelaxation were measured in aortas of wild-type mice or mice deficient for nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase subunits (p47(phox) or gp91(phox)) or indoleamine-pyrrole 2,3-dioxygenase 1 with or without angiotensin (Ang) II infusion. As expected, AngII increased plasma levels of Kyn- and 3-hydroxykynurenine-modified proteins in endothelial cells in vivo. Consistent with this, AngII markedly increased the expression of indoleamine-pyrrole 2,3-dioxygenase in parallel with increased expression of interferon-γ. Furthermore, in wild-type mice, AngII significantly increased oxidative stress, endothelial cell apoptosis, and endothelial dysfunction. These effects of AngII infusion were significantly suppressed in mice deficient for p47(phox), gp91(phox), or indoleamine-pyrrole 2,3-dioxygenase 1, suggesting that AngII-induced enhancement of Kynurenines via NAD(P)H oxidase-derived oxidants causes endothelial cell apoptosis and dysfunction in vivo. Furthermore, interferon-γ neutralization eliminates AngII-increased superoxide products and endothelial apoptosis by inhibiting AngII-induced Kynurenines generation, suggesting that AngII-activated Kyn pathway is interferon-γ-dependent. Mechanistically, we found that AngII-enhanced 3-hydroxykynurenine promoted the generation of NAD(P)H oxidase-mediated superoxide anions by increasing the translocation and membrane assembly of NAD(P)H oxidase subunits in endothelial cells, resulting in accelerated apoptosis and consequent endothelial dysfunction.
Conclusions: Kyn pathway activation accelerates apoptosis and dysfunction of the endothelium by upregulating NAD(P)H-derived superoxide.
Keywords: 3-hydroxykynurenine; NAD(P)H oxidase; apoptosis; indoleamine-pyrrole 2,3,-dioxygenase; kynurenine; oxidative stress; tryptophan.
Figures



Comment in
-
Do endothelial cells eat tryptophan to die?Circ Res. 2014 Jan 31;114(3):406-8. doi: 10.1161/CIRCRESAHA.113.303150. Circ Res. 2014. PMID: 24481839 Free PMC article. No abstract available.
References
-
- Stefanec T. Endothelial apoptosis: Could it have a role in the pathogenesis and treatment of disease? Chest. 2000;117:841–854. - PubMed
-
- Galley HF, Webster NR. Physiology of the endothelium. British journal of anaesthesia. 2004;93:105–113. - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circulation research. 2000;87:840–844. - PubMed
-
- King NJ, Thomas SR. Molecules in focus: Indoleamine 2,3-dioxygenase. The international journal of biochemistry & cell biology. 2007;39:2167–2172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL074399/HL/NHLBI NIH HHS/United States
- HL080499/HL/NHLBI NIH HHS/United States
- R01 AG047776/AG/NIA NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- HL096032/HL/NHLBI NIH HHS/United States
- HL110488/HL/NHLBI NIH HHS/United States
- R01 HL089920/HL/NHLBI NIH HHS/United States
- R01 HL074399/HL/NHLBI NIH HHS/United States
- HL089920/HL/NHLBI NIH HHS/United States
- HL079584/HL/NHLBI NIH HHS/United States
- HL105157/HL/NHLBI NIH HHS/United States
- R01 HL110488/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

