Silencing of RB1 and RB2/P130 during adipogenesis of bone marrow stromal cells results in dysregulated differentiation
- PMID: 24281253
- PMCID: PMC3956544
- DOI: 10.4161/cc.27275
Silencing of RB1 and RB2/P130 during adipogenesis of bone marrow stromal cells results in dysregulated differentiation
Erratum in
-
Correction.Cell Cycle. 2020 Aug;19(16):2081. doi: 10.1080/15384101.2020.1795491. Epub 2020 Jul 17. Cell Cycle. 2020. PMID: 32677534 Free PMC article. No abstract available.
Abstract
Bone marrow adipose tissue (BMAT) is different from fat found elsewhere in the body, and only recently have some of its functions been investigated. BMAT may regulate bone marrow stem cell niche and plays a role in energy storage and thermogenesis. BMAT may be involved also in obesity and osteoporosis onset. Given the paramount functions of BMAT, we decided to better clarify the human bone marrow adipogenesis by analyzing the role of the retinoblastoma gene family, which are key players in cell cycle regulation. Our data provide evidence that the inactivation of RB1 or RB2/P130 in uncommitted bone marrow stromal cells (BMSC) facilitates the first steps of adipogenesis. In cultures with silenced RB1 or RB2/P130, we observed an increase of clones with adipogenic potential and a higher percentage of cells accumulating lipid droplets. Nevertheless, the absence of RB1 or RB2/P130 impaired the terminal adipocyte differentiation and gave rise to dysregulated adipose cells, with alteration in lipid uptake and release. For the first time, we evidenced that RB2/P130 plays a role in bone marrow adipogenesis. Our data suggest that while the inactivation of retinoblastoma proteins may delay the onset of last cell division and allow more BMSC to be committed to adipocyte, it did not allow a permanent cell cycle exit, which is a prerequisite for adipocyte terminal maturation.
Keywords: adipocytes; bone marrow; differentiation; marrow stromal stem cells; retinoblastoma gene family.
Figures

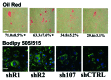
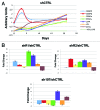
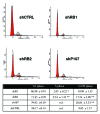
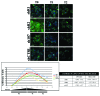
Comment in
-
RB orchestrates fat cell and cell fate.Cell Cycle. 2014;13(4):508. doi: 10.4161/cc.27865. Epub 2014 Jan 22. Cell Cycle. 2014. PMID: 24496330 No abstract available.
References
-
- Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol 2006:249-82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
