Chaperone-mediated autophagy: roles in disease and aging
- PMID: 24281265
- PMCID: PMC3879702
- DOI: 10.1038/cr.2013.153
Chaperone-mediated autophagy: roles in disease and aging
Abstract
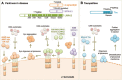
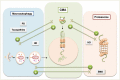
This review focuses on chaperone-mediated autophagy (CMA), one of the proteolytic systems that contributes to degradation of intracellular proteins in lysosomes. CMA substrate proteins are selectively targeted to lysosomes and translocated into the lysosomal lumen through the coordinated action of chaperones located at both sides of the membrane and a dedicated protein translocation complex. The selectivity of CMA permits timed degradation of specific proteins with regulatory purposes supporting a modulatory role for CMA in enzymatic metabolic processes and subsets of the cellular transcriptional program. In addition, CMA contributes to cellular quality control through the removal of damaged or malfunctioning proteins. Here, we describe recent advances in the understanding of the molecular dynamics, regulation and physiology of CMA, and discuss the evidence in support of the contribution of CMA dysfunction to severe human disorders such as neurodegeneration and cancer.
Figures

References
-
- Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15:305–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

