Different iron sources to study the physiology and biochemistry of iron metabolism in marine micro-algae
- PMID: 24281777
- PMCID: PMC3905174
- DOI: 10.1007/s10534-013-9688-1
Different iron sources to study the physiology and biochemistry of iron metabolism in marine micro-algae
Abstract
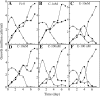
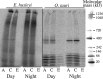
We compared ferric EDTA, ferric citrate and ferrous ascorbate as iron sources to study iron metabolism in Ostreococcus tauri, Phaeodactlylum tricornutum and Emiliania huxleyi. Ferric EDTA was a better iron source than ferric citrate for growth and chlorophyll levels. Direct and indirect experiments showed that iron was much more available to the cells when provided as ferric citrate as compared to ferric EDTA. As a consequence, growth media with iron concentration in the range 1-100 nM were rapidly iron-depleted when ferric citrate-but not ferric EDTA was the iron source. When cultured together, P. tricornutum cells overgrew the two other species in iron-sufficient conditions, but E. huxleyi was able to compete other species in iron-deficient conditions, and when iron was provided as ferric citrate instead of ferric EDTA, which points out the critical influence of the chemical form of iron on the blooms of some phytoplankton species. The use of ferric citrate and ferrous ascorbate allowed us to unravel a kind of regulation of iron uptake that was dependent on the day/night cycles and to evidence independent uptake systems for ferrous and ferric iron, which can be regulated independently and be copper-dependent or independent. The same iron sources also allowed one to identify molecular components involved in iron uptake and storage in marine micro-algae. Characterizing the mechanisms of iron metabolism in the phytoplankton constitutes a big challenge; we show here that the use of iron sources more readily available to the cells than ferric EDTA is critical for this task.
Figures




Similar articles
-
Ostreococcus tauri is a new model green alga for studying iron metabolism in eukaryotic phytoplankton.BMC Genomics. 2016 May 3;17:319. doi: 10.1186/s12864-016-2666-6. BMC Genomics. 2016. PMID: 27142620 Free PMC article.
-
A comparative study of iron uptake mechanisms in marine microalgae: iron binding at the cell surface is a critical step.Plant Physiol. 2012 Dec;160(4):2271-84. doi: 10.1104/pp.112.204156. Epub 2012 Oct 2. Plant Physiol. 2012. PMID: 23033141 Free PMC article.
-
The mammalian transferrin-independent iron transport system may involve a surface ferrireductase activity.Biochem J. 1994 Sep 15;302 ( Pt 3)(Pt 3):875-9. doi: 10.1042/bj3020875. Biochem J. 1994. PMID: 7945215 Free PMC article.
-
The potential role of NaFeEDTA as an iron fortificant.Int J Vitam Nutr Res. 2004 Nov;74(6):421-34. doi: 10.1024/0300-9831.74.6.421. Int J Vitam Nutr Res. 2004. PMID: 15743018 Review.
-
Iron and citric acid: a fuzzy chemistry of ubiquitous biological relevance.Biometals. 2000 Mar;13(1):91-6. doi: 10.1023/a:1009225701332. Biometals. 2000. PMID: 10831230 Review.
Cited by
-
Ostreococcus tauri is a new model green alga for studying iron metabolism in eukaryotic phytoplankton.BMC Genomics. 2016 May 3;17:319. doi: 10.1186/s12864-016-2666-6. BMC Genomics. 2016. PMID: 27142620 Free PMC article.
-
Effect of Metals, Metalloids and Metallic Nanoparticles on Microalgae Growth and Industrial Product Biosynthesis: A Review.Int J Mol Sci. 2015 Oct 9;16(10):23929-69. doi: 10.3390/ijms161023929. Int J Mol Sci. 2015. PMID: 26473834 Free PMC article. Review.
-
Comparative study of multiple approaches for identifying cultivable microalgae population diversity from freshwater samples.PLoS One. 2023 Jul 7;18(7):e0285913. doi: 10.1371/journal.pone.0285913. eCollection 2023. PLoS One. 2023. PMID: 37418475 Free PMC article.
-
Acclimation of a low iron adapted Ostreococcus strain to iron limitation through cell biomass lowering.Sci Rep. 2017 Mar 23;7(1):327. doi: 10.1038/s41598-017-00216-6. Sci Rep. 2017. PMID: 28336917 Free PMC article.
-
Central role for ferritin in the day/night regulation of iron homeostasis in marine phytoplankton.Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):14652-7. doi: 10.1073/pnas.1506074112. Epub 2015 Nov 9. Proc Natl Acad Sci U S A. 2015. PMID: 26553998 Free PMC article.
References
-
- Allen MD, del Campo JA, Kropat J, Merchant SS. FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryot Cell. 2007;6:1841–1852. doi: 10.1128/EC.00205-07. - DOI - PMC - PubMed
-
- Anderson MA, Morel FMM. The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol Oceanogr. 1982;27:789–813. doi: 10.4319/lo.1982.27.5.0789. - DOI
-
- Blaiseau P-L, Seguin A, Camadro JM, Lesuisse E. Iron uptake in yeasts. In: Cornelis P, Andrews SC, editors. Iron uptake and homeostasis in microorganisms. Brussels & Reading: Caister Academic Press; 2010. pp. 265–284.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

