Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties
- PMID: 24281828
- PMCID: PMC3889836
- DOI: 10.1007/s10549-013-2779-4
Prostaglandin E receptor EP4 is a therapeutic target in breast cancer cells with stem-like properties
Abstract
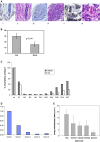
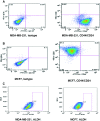
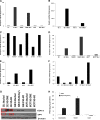
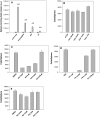
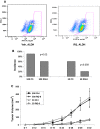
The cyclooxygenase pathway is strongly implicated in breast cancer progression but the role of this pathway in the biology of breast cancer stem/progenitor cells has not been defined. Recent attention has focused on targeting the cyclooxygenase 2 (COX-2) pathway downstream of the COX-2 enzyme by blocking the activities of individual prostaglandin E (EP) receptors. Prostaglandin E receptor 4 (EP4) is widely expressed in primary invasive ductal carcinomas of the breast and antagonizing this receptor with small molecule inhibitors or shRNA directed to EP4 inhibits metastatic potential in both syngeneic and xenograft models. Breast cancer stem/progenitor cells are defined as a subpopulation of cells that drive tumor growth, metastasis, treatment resistance, and relapse. Mammosphere-forming breast cancer cells of human (MDA-MB-231, SKBR3) or murine (66.1, 410.4) origin of basal-type, Her-2 phenotype and/or with heightened metastatic capacity upregulate expression of both EP4 and COX-2 and are more tumorigenic compared to the bulk population. In contrast, luminal-type or non-metastatic counterparts (MCF7, 410, 67) do not increase COX-2 and EP4 expression in mammosphere culture. Treatment of mammosphere-forming cells with EP4 inhibitors (RQ-15986, AH23848, Frondoside A) or EP4 gene silencing, but not with a COX inhibitor (Indomethacin) reduces both mammosphere-forming capacity and the expression of phenotypic markers (CD44(hi)/CD24(low), aldehyde dehydrogenase) of breast cancer stem cells. Finally, an orally delivered EP4 antagonist (RQ-08) reduces the tumor-initiating capacity and markedly inhibits both the size of tumors arising from transplantation of mammosphere-forming cells and phenotypic markers of stem cells in vivo. These studies support the continued investigation of EP4 as a potential therapeutic target and provide new insight regarding the role of EP4 in supporting a breast cancer stem cell/tumor-initiating phenotype.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. - PubMed
-
- Mutoh M, Watanabe K, Kitamura T, Shoji Y, Takahashi M, Kawamori T, et al. Involvement of prostaglandin E receptor subtype EP4 in colon carcinogenesis. Cancer Res. 2002;62:28–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

