A dendritic thioester hydrogel based on thiol-thioester exchange as a dissolvable sealant system for wound closure
- PMID: 24282150
- PMCID: PMC4000691
- DOI: 10.1002/anie.201308007
A dendritic thioester hydrogel based on thiol-thioester exchange as a dissolvable sealant system for wound closure
Abstract
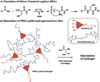
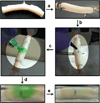
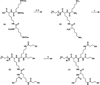
A dissolvable dendritic thioester hydrogel based on thiol-thioester exchange for wound closure is reported. The hydrogel sealant adheres strongly to tissues, closes an ex vivo vein puncture, and withstands high pressures placed on a wound. The hydrogel sealant can be completely washed off upon exposure to thiolates based on thiol-thioester exchange and allow gradual wound re-exposure during definitive surgical care.
Keywords: dendrimers; hemostasis; hydrogels; supramolecular chemistry; wound dressing.
Figures
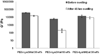


References
-
- Bracher PJ, Snyder PW, Bohall BR, Whitesides GM. Origins of Life and Evolution of Biospheres. 2011;41:399. - PubMed
-
- Ura Y, Beierle JM, Leman LJ, Orgel LE, Ghadiri MR. Science. 2009;325:73. - PubMed
-
- Kopecek J, Poly J. Sci. Part A: Polymer Chemistry. 2009;47:5929. - PMC - PubMed
- Zhao F, Ma ML, Xu B. Chem. Soc. Rev. 2009;38:883. - PubMed
- Qiu Y, Park K. Adv Drug. Del. Rev. 2012;64:49060.
- Hennink WE, van Nostrum CF. Adv. Drig. Del. Rev. 2012;64:223.
- Guvendiren M, Lu HD, Burdick JA. Soft Matter. 2012;8:260.
- Krishna OD, Kiick KL. Biopolymers. 2010;94:32. - PMC - PubMed
- Hoffman AS. Adv. Drug. Del. Rev. 2013;65:10. - PubMed
- Luo Z, Wang S, Zhang S. Biomaterials. 2011;32:2013. - PubMed
-
- Alam HB, Burris D, DaCorta JA, Rhee P. Military medicine. 2005;170:63. - PubMed
-
- Wathier M, Johnson SM, Kim T, Grinstaff MW. Bioconjugate Chem. 2006;17:873. - PubMed
- Hu B-H, Su J, Messersmith PB. Biomacromolecules. 2009;10:2194. - PMC - PubMed
- Anumolu SS, Menjoge AR, Deshmukh M, Gerecke D, Stein S, Laskin J, Sinko PJ. Biomaterials. 2011;32:1204. - PMC - PubMed
- Strehin I, Gourevitch D, Zhang Y, Heber-Katz E, Messersmith PB. Biomaterials Science. 2013;1:603. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

