eRapa restores a normal life span in a FAP mouse model
- PMID: 24282255
- PMCID: PMC4058993
- DOI: 10.1158/1940-6207.CAPR-13-0299
eRapa restores a normal life span in a FAP mouse model
Abstract
Mutation of a single copy of the adenomatous polyposis coli (APC) gene results in familial adenomatous polyposis (FAP), which confers an extremely high risk for colon cancer. Apc(Min/+) mice exhibit multiple intestinal neoplasia (MIN) that causes anemia and death from bleeding by 6 months. Mechanistic target of rapamycin complex 1 (mTORC1) inhibitors were shown to improve Apc(Min/+) mouse survival when administered by oral gavage or added directly to the chow, but these mice still died from neoplasia well short of a natural life span. The National Institute of Aging Intervention Testing Program showed that enterically targeted rapamycin (eRapa) extended life span for wild-type genetically heterogeneous mice in part by inhibiting age-associated cancer. We hypothesized that eRapa would be effective in preventing neoplasia and extend survival of Apc(Min/+) mice. We show that eRapa improved survival of Apc(Min/+) mice in a dose-dependent manner. Remarkably, and in contrast to previous reports, most of the Apc(Min/+) mice fed 42 parts per million eRapa lived beyond the median life span reported for wild-type syngeneic mice. Furthermore, chronic eRapa did not cause detrimental immune effects in mouse models of cancer, infection, or autoimmunity, thus assuaging concerns that chronic rapamycin treatment suppresses immunity. Our studies suggest that a novel formulation (enteric targeting) of a well-known and widely used drug (rapamycin) can dramatically improve its efficacy in targeted settings. eRapa or other mTORC1 inhibitors could serve as effective cancer preventatives for people with FAP without suppressing the immune system, thus reducing the dependency on surgery as standard therapy.
©2013 AACR.
Conflict of interest statement
ZDS and RS are unpaid Scientific Advisory Board members for Rapamycin Holdings, Inc. The remaining authors do not have any relationships that could be construed as resulting in an actual, potential, or perceived conflict of interest with regard to the submitted manuscript.
Figures
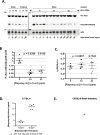

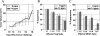

References
-
- Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Annals of Internal Medicine. 2011;154:22–30. - PubMed
-
- Wallace MH, Phillips RK. Upper gastrointestinal disease in patients with familial adenomatous polyposis. The British journal of surgery. 1998;85:742–50. - PubMed
-
- Hasty P. Rapamycin: The Cure for all that Ails. J Mol Cell Biol. 2010;2:17–9. - PubMed
-
- Piselli P, Serraino D, Segoloni GP, Sandrini S, Piredda GB, Scolari MP, et al. Risk of de novo cancers after transplantation: Results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. European Journal of Cancer. 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

