Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia
- PMID: 24282296
- PMCID: PMC3864280
- DOI: 10.1073/pnas.1314197110
Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia
Abstract
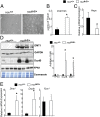
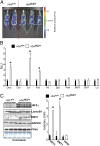
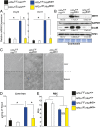
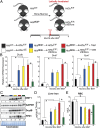
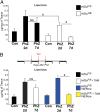
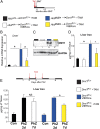
Several distinct congenital disorders can lead to tissue-iron overload with anemia. Repeated blood transfusions are one of the major causes of iron overload in several of these disorders, including β-thalassemia major, which is characterized by a defective β-globin gene. In this state, hyperabsorption of iron is also observed and can significantly contribute to iron overload. In β-thalassemia intermedia, which does not require blood transfusion for survival, hyperabsorption of iron is the leading cause of iron overload. The mechanism of increased iron absorption in β-thalassemia is unclear. We definitively demonstrate, using genetic mouse models, that intestinal hypoxia-inducible factor-2α (HIF2α) and divalent metal transporter-1 (DMT1) are activated early in the pathogenesis of β-thalassemia and are essential for excess iron accumulation in mouse models of β-thalassemia. Moreover, thalassemic mice with established iron overload had significant improvement in tissue-iron levels and anemia following disruption of intestinal HIF2α. In addition to repeated blood transfusions and increased iron absorption, chronic hemolysis is the major cause of tissue-iron accumulation in anemic iron-overload disorders caused by hemolytic anemia. Mechanistic studies in a hemolytic anemia mouse model demonstrated that loss of intestinal HIF2α/DMT1 signaling led to decreased tissue-iron accumulation in the liver without worsening the anemia. These data demonstrate that dysregulation of intestinal hypoxia and HIF2α signaling is critical for progressive iron overload in β-thalassemia and may be a novel therapeutic target in several anemic iron-overload disorders.
Keywords: Epas1; HIF; Slc11a2; thalassemia.
Conflict of interest statement
Conflict of interest statement: S. Rivella is a consultant for Exigo Management Consultant, Isis, Bayer AG, BioMarin, Merganser Biotech, and Novartis Pharmaceuticals. He also holds an equity/ownership interest in Merganser Biotech, Inc. In addition, he is a coinventor for the patents US8058061 B2 C12N 20111115 and US7541179 B2 C12N 20090602. The consulting work and intellectual property of S. Rivella did not affect in any way the design, conduct, or reporting of this research.
Figures

References
-
- Steinberg MHFB, Higgs DR, Nagel RL. Disorders of Hemoglobin: Genetics, Pathophysiology and Clincal Management. Cambridge, UK: Cambridge Univ Press; 2001.
-
- Heinrich HC, et al. Absorption of inorganic and food iron in children with heterozygous and homozygous beta-thalassemia. Z Kinderheilkd. 1973;115(1):1–22. - PubMed
-
- Pitcher CS, Williams HS, Parsonson A, Williams R. The measurement of iron absorption by the double isotope technique. Br J Haematol. 1965;11(6):633–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

