Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation
- PMID: 24282306
- PMCID: PMC3864306
- DOI: 10.1073/pnas.1313654110
Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation
Abstract
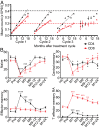
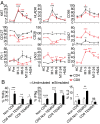
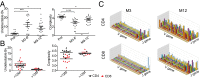
The association between lymphopenia and autoimmunity is recognized, but the underlying mechanisms are poorly understood and have not been studied systematically in humans. People with multiple sclerosis treated with the lymphocyte-depleting monoclonal antibody alemtuzumab offer a unique opportunity to study this phenomenon; one in three people develops clinical autoimmunity, and one in three people develops asymptomatic autoantibodies after treatment. Here, we show that T-cell recovery after alemtuzumab is driven by homeostatic proliferation, leading to the generation of chronically activated (CD28(-)CD57(+)), highly proliferative (Ki67(+)), oligoclonal, memory-like CD4 and CD8 T cells (CCR7(-)CD45RA(-) or CCR7(-)CD45RA(+)) capable of producing proinflammatory cytokines. Individuals who develop autoimmunity after treatment are no more lymphopenic than their nonautoimmune counterparts, but they show reduced thymopoiesis and generate a more restricted T-cell repertoire. Taken together, these findings demonstrate that homeostatic proliferation drives lymphopenia-associated autoimmunity in humans.
Keywords: T lymphocytes; immunotherapy; reconstitution.
Conflict of interest statement
Conflict of interest statement: J.L.J., A.C., and A.J. Coles report receiving consulting and lecture fees from Genzyme. A.C. and A.J. Coles report research support paid to their institution from Genzyme. No other authors report competing interests.
Figures



References
-
- Cohen JA, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819–1828. - PubMed
-
- Coles AJ, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786–1801. - PubMed
-
- Coles AJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839. - PubMed
-
- Hill-Cawthorne GA, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83(3):298–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

