Cytokine Spatzle binds to the Drosophila immunoreceptor Toll with a neurotrophin-like specificity and couples receptor activation
- PMID: 24282309
- PMCID: PMC3870763
- DOI: 10.1073/pnas.1317002110
Cytokine Spatzle binds to the Drosophila immunoreceptor Toll with a neurotrophin-like specificity and couples receptor activation
Abstract
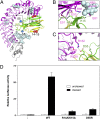
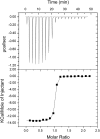
Drosophila Toll functions in embryonic development and innate immunity and is activated by an endogenous ligand, Spätzle (Spz). The related Toll-like receptors in vertebrates also function in immunity but are activated directly by pathogen-associated molecules such as bacterial endotoxin. Here, we present the crystal structure at 2.35-Å resolution of dimeric Spz bound to a Toll ectodomain encompassing the first 13 leucine-rich repeats. The cystine knot of Spz binds the concave face of the Toll leucine-rich repeat solenoid in an area delineated by N-linked glycans and induces a conformational change. Mutagenesis studies confirm that the interface observed in the crystal structure is relevant for signaling. The asymmetric binding mode of Spz to Toll is similar to that of nerve growth factor (NGF) in complex with the p75 neurotrophin receptor but is distinct from that of microbial ligands bound to the Toll-like receptors. Overall, this study indicates an allosteric signaling mechanism for Toll in which ligand binding to the N terminus induces a conformational change that couples to homodimerization of juxtamembrane structures in the Toll ectodomain C terminus.
Keywords: Spz ligand; Toll receptor; crystallography; isothermal titration calorimetry; mass spectrometry.
Conflict of interest statement
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank,
Figures



Similar articles
-
Structure of the Toll-Spatzle complex, a molecular hub in Drosophila development and innate immunity.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6281-6. doi: 10.1073/pnas.1320678111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733933 Free PMC article.
-
Structural insight into the mechanism of activation of the Toll receptor by the dimeric ligand Spätzle.J Biol Chem. 2008 May 23;283(21):14629-35. doi: 10.1074/jbc.M800112200. Epub 2008 Mar 17. J Biol Chem. 2008. PMID: 18347020
-
Functional insights from the crystal structure of the N-terminal domain of the prototypical toll receptor.Structure. 2013 Jan 8;21(1):143-153. doi: 10.1016/j.str.2012.11.003. Epub 2012 Dec 13. Structure. 2013. PMID: 23245851 Free PMC article.
-
The Drosophila Toll signaling pathway.J Immunol. 2011 Jan 15;186(2):649-56. doi: 10.4049/jimmunol.1002302. J Immunol. 2011. PMID: 21209287 Review.
-
Structures and recognition modes of toll-like receptors.Proteins. 2017 Jan;85(1):3-9. doi: 10.1002/prot.25179. Epub 2016 Oct 24. Proteins. 2017. PMID: 27699870 Review.
Cited by
-
Drosophila as a Model for Human Viral Neuroinfections.Cells. 2022 Aug 29;11(17):2685. doi: 10.3390/cells11172685. Cells. 2022. PMID: 36078091 Free PMC article. Review.
-
Structure of the Toll-Spatzle complex, a molecular hub in Drosophila development and innate immunity.Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6281-6. doi: 10.1073/pnas.1320678111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733933 Free PMC article.
-
The COP II adaptor protein TMED7 is required to initiate and mediate the delivery of TLR4 to the plasma membrane.Sci Signal. 2014 Jul 29;7(336):ra70. doi: 10.1126/scisignal.2005275. Sci Signal. 2014. PMID: 25074978 Free PMC article.
-
Structure and dynamics of Toll immunoreceptor activation in the mosquito Aedes aegypti.Nat Commun. 2022 Aug 30;13(1):5110. doi: 10.1038/s41467-022-32690-6. Nat Commun. 2022. PMID: 36042238 Free PMC article.
-
Immune-Related Gene Profiles and Differential Expression in the Grey Garden Slug Deroceras reticulatum Infected with the Parasitic Nematode Phasmarhabditis hermaphrodita.Insects. 2024 Apr 26;15(5):311. doi: 10.3390/insects15050311. Insects. 2024. PMID: 38786867 Free PMC article.
References
-
- Anderson KV, Bokla L, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: The induction of polarity by the Toll gene product. Cell. 1985;42(3):791–798. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. - PubMed
-
- Rosetto M, Engström Y, Baldari CT, Telford JL, Hultmark D. Signals from the IL-1 receptor homolog, Toll, can activate an immune response in a Drosophila hemocyte cell line. Biochem Biophys Res Commun. 1995;209(1):111–116. - PubMed
-
- Kaneko T, et al. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20(5):637–649. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

