Plant cytoplasmic GAPDH: redox post-translational modifications and moonlighting properties
- PMID: 24282406
- PMCID: PMC3824636
- DOI: 10.3389/fpls.2013.00450
Plant cytoplasmic GAPDH: redox post-translational modifications and moonlighting properties
Abstract
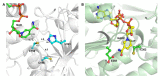
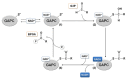
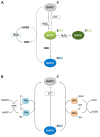
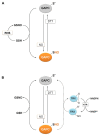
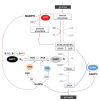
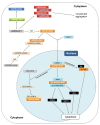
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a ubiquitous enzyme involved in glycolysis and shown, particularly in animal cells, to play additional roles in several unrelated non-metabolic processes such as control of gene expression and apoptosis. This functional versatility is regulated, in part at least, by redox post-translational modifications that alter GAPDH catalytic activity and influence the subcellular localization of the enzyme. In spite of the well established moonlighting (multifunctional) properties of animal GAPDH, little is known about non-metabolic roles of GAPDH in plants. Plant cells contain several GAPDH isoforms with different catalytic and regulatory properties, located both in the cytoplasm and in plastids, and participating in glycolysis and the Calvin-Benson cycle. A general feature of all GAPDH proteins is the presence of an acidic catalytic cysteine in the active site that is overly sensitive to oxidative modifications, including glutathionylation and S-nitrosylation. In Arabidopsis, oxidatively modified cytoplasmic GAPDH has been successfully used as a tool to investigate the role of reduced glutathione, thioredoxins and glutaredoxins in the control of different types of redox post-translational modifications. Oxidative modifications inhibit GAPDH activity, but might enable additional functions in plant cells. Mounting evidence support the concept that plant cytoplasmic GAPDH may fulfill alternative, non-metabolic functions that are triggered by redox post-translational modifications of the protein under stress conditions. The aim of this review is to detail the molecular mechanisms underlying the redox regulation of plant cytoplasmic GAPDH in the light of its crystal structure, and to provide a brief inventory of the well known redox-dependent multi-facetted properties of animal GAPDH, together with the emerging roles of oxidatively modified GAPDH in stress signaling pathways in plants.
Keywords: S-nitrosylation; cysteine thiols; glycolytic glyceraldehyde-3-phosphate dehydrogenase; moonlighting protein; redox modifications.
Figures
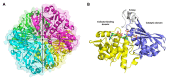


References
-
- Anderson L. E., Bryant J. A., Carol A. A. (2004). Both chloroplastic and cytosolic phosphoglycerate kinase isozymes are present in the pea leaf nucleus. Protoplasma 223 103–110 - PubMed
-
- Bedhomme M., Adamo M., Marchand C. H., Couturier J., Rouhier N., Lemaire S. D., et al. (2012). Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. Biochem. J. 445 337–347 10.1042/BJ20120505 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

