Platelets and erythrocyte-bound platelets bind infectious HIV-1 in plasma of chronically infected patients
- PMID: 24282562
- PMCID: PMC3839895
- DOI: 10.1371/journal.pone.0081002
Platelets and erythrocyte-bound platelets bind infectious HIV-1 in plasma of chronically infected patients
Abstract
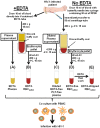
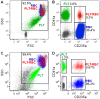
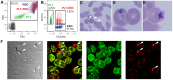
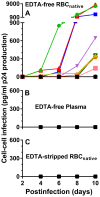
Chronic HIV-1 infection is associated with persistent viremia in most patients, but it remains unclear how free virus may survive the potential hostile effects of plasma. We investigated whether sites might exist on the surfaces of circulating blood cells for protection of infectious HIV-1 particles. Red blood cells (RBC) either from blood of uninfected normal individuals, or from blood obtained without EDTA from chronically infected HIV-1 patients, invariably contained a small number of RBC having attached platelets as determined by flow cytometry, light microscopy, and immunofluorescence microscopy. After mixing normal RBC with platelet-rich plasma, discrete populations of RBC, platelets, and complexes of platelets attached to RBC were purified by fluorescence-activated cell sorting. Upon incubation of purified cells or platelets with HIV-1 followed by washing and co-incubation with CD4-positive peripheral blood mononuclear cells (PBMC), platelets, and platelet-RBC complexes, but not platelet-free RBC, caused infection of PBMC. Infection was prevented by pre-treating the platelet-RBC complexes with EDTA. Plasma and RBC (comprising a RBC/platelet-RBC mixture) from chronically infected patients with low viral loads were also co-incubated with PBMC ex vivo to determine the presence of infectious HIV-1. All freshly isolated plasmas from the HIV-1-infected donors, obtained in the absence of anticoagulant, were noninfectious. Interestingly, the RBC from most of the patients caused cell-cell infection of PBMC that was prevented by stripping the RBC with EDTA. A monoclonal antibody to DC-SIGN partially inhibited cell-cell HIV-1 infection of PBMC by normal RBC pre-incubated with platelets and HIV-1. We conclude: (a) platelet-free EDTA-free plasma from chronically infected HIV-1 patients, although containing viral RNA, is an environment that lacks detectable infectious HIV-1; (b) platelets and platelet-RBC complexes, but not purified RBC, bind infectious HIV-1; (c) DC-SIGN, and possibly other C-type lectins, may represent binding sites for infectious HIV-1 on platelets and platelet-RBC complexes.
Conflict of interest statement
Figures



Similar articles
-
Platelets in HIV: A Guardian of Host Defence or Transient Reservoir of the Virus?Front Immunol. 2021 Apr 23;12:649465. doi: 10.3389/fimmu.2021.649465. eCollection 2021. Front Immunol. 2021. PMID: 33968041 Free PMC article. Review.
-
Distribution of HIV type 1 (HIV-1) in blood components: detection and significance of high levels of HIV-1 associated with platelets.Transfusion. 1998 Jun;38(6):580-8. doi: 10.1046/j.1537-2995.1998.38698326338.x. Transfusion. 1998. PMID: 9661692 Clinical Trial.
-
DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets.J Virol. 2006 Sep;80(18):8951-60. doi: 10.1128/JVI.00136-06. J Virol. 2006. PMID: 16940507 Free PMC article.
-
Human erythrocytes selectively bind and enrich infectious HIV-1 virions.PLoS One. 2009 Dec 14;4(12):e8297. doi: 10.1371/journal.pone.0008297. PLoS One. 2009. PMID: 20011536 Free PMC article.
-
[Single-donor (apheresis) platelets and pooled whole-blood-derived platelets--significance and assessment of both blood products].Clin Lab. 2014;60(4):S1-39. doi: 10.7754/clin.lab.2014.140210. Clin Lab. 2014. PMID: 24779310 Review. German.
Cited by
-
Thrombocytopenia in Virus Infections.J Clin Med. 2021 Feb 20;10(4):877. doi: 10.3390/jcm10040877. J Clin Med. 2021. PMID: 33672766 Free PMC article. Review.
-
Platelets kill circulating parasites of all major Plasmodium species in human malaria.Blood. 2018 Sep 20;132(12):1332-1344. doi: 10.1182/blood-2018-05-849307. Epub 2018 Jul 19. Blood. 2018. PMID: 30026183 Free PMC article.
-
Platelets in HIV: A Guardian of Host Defence or Transient Reservoir of the Virus?Front Immunol. 2021 Apr 23;12:649465. doi: 10.3389/fimmu.2021.649465. eCollection 2021. Front Immunol. 2021. PMID: 33968041 Free PMC article. Review.
-
From Classical to Unconventional: The Immune Receptors Facilitating Platelet Responses to Infection and Inflammation.Biology (Basel). 2020 Oct 20;9(10):343. doi: 10.3390/biology9100343. Biology (Basel). 2020. PMID: 33092021 Free PMC article. Review.
-
HIV Latency in Myeloid Cells: Challenges for a Cure.Pathogens. 2022 May 24;11(6):611. doi: 10.3390/pathogens11060611. Pathogens. 2022. PMID: 35745465 Free PMC article. Review.
References
-
- Piatak M Jr, Saag MS, Yang LC, Clark SJ, Kappes JC, et al. (1993) High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259(5102): 1749–1754. - PubMed
-
- Palmer S, Josefsson L, Coffin JM (2011) HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med 270(6): 550–560. - PubMed
-
- Chun TW, Fauci AS (2012) HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS 26(10): 1261–1268. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

