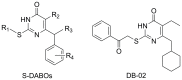
DB-02, a C-6-cyclohexylmethyl substituted pyrimidinone HIV-1 reverse transcriptase inhibitor with nanomolar activity, displays an improved sensitivity against K103N or Y181C than S-DABOs
- PMID: 24282600
- PMCID: PMC3839930
- DOI: 10.1371/journal.pone.0081489
DB-02, a C-6-cyclohexylmethyl substituted pyrimidinone HIV-1 reverse transcriptase inhibitor with nanomolar activity, displays an improved sensitivity against K103N or Y181C than S-DABOs
Abstract
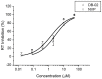
6-(cyclohexylmethyl)-5-ethyl-2-((2-oxo-2-phenylethyl)thio)pyrimidin-4(3H)-one (DB-02) is a member of the newly reported synthetic anti-HIV-1 compounds dihydro-aryl/alkylsulfanyl-cyclohexylmethyl-oxopyrimidines, S-DACOs. In vitro anti-HIV-1 activity and resistance profile studies have suggested that DB-02 has very low cytotoxicity (CC50>1mM) to cell lines and peripheral blood mononuclear cells (PBMCs). It displays potent anti-HIV-1 activity against laboratory adapted strains and primary isolated strains including different subtypes and tropism strains (EC50s range from 2.40 to 41.8 nM). Studies on site-directed mutagenesis, genotypic resistance profiles revealed that V106A was the major resistance contributor for the compound. Molecular docking analysis showed that DB-02 located in the hydrophobic pocket with interactions of Lys101, Val106, Leu234, His235. DB-02 also showed non-antagonistic effects to four approved antiretroviral drugs. All studies indicated that DB-02 would be a potential NNRTI with low cytotoxicity and improved activity.
Conflict of interest statement
Figures


Similar articles
-
Computer-aided design, synthesis, and anti-HIV-1 activity in vitro of 2-alkylamino-6-[1-(2,6-difluorophenyl)alkyl]-3,4-dihydro-5-alkylpyrimidin-4(3H)-ones as novel potent non-nucleoside reverse transcriptase inhibitors, also active against the Y181C variant.J Med Chem. 2004 Feb 12;47(4):928-34. doi: 10.1021/jm0309856. J Med Chem. 2004. PMID: 14761194
-
Structure-based design, synthesis, and biological evaluation of conformationally restricted novel 2-alkylthio-6-[1-(2,6-difluorophenyl)alkyl]-3,4-dihydro-5-alkylpyrimidin-4(3H)-ones as non-nucleoside inhibitors of HIV-1 reverse transcriptase.J Med Chem. 2001 Aug 2;44(16):2544-54. doi: 10.1021/jm010853h. J Med Chem. 2001. PMID: 11472208
-
Nonnucleoside HIV-1 reverse transcriptase inhibitors; part 3. Synthesis and antiviral activity of 5-alkyl-2-[(aryl and alkyloxyl-carbonylmethyl)thio]-6-(1-naphthylmethyl) pyrimidin-4(3H)-ones.Bioorg Chem. 2004 Dec;32(6):536-48. doi: 10.1016/j.bioorg.2004.05.007. Bioorg Chem. 2004. PMID: 15530994
-
Non-nucleoside HIV reverse transcriptase inhibitors, Part 6[1]: synthesis and anti-HIV activity of novel 2-[(arylcarbonylmethyl)thio]-6-arylthio DABO analogues.Arch Pharm (Weinheim). 2005 Oct;338(10):457-61. doi: 10.1002/ardp.200400961. Arch Pharm (Weinheim). 2005. PMID: 16211654
-
Recent advances in the DABOs family as potent HIV-1 non-nucleoside reverse transcriptase inhibitors.Curr Med Chem. 2011;18(16):2376-85. doi: 10.2174/092986711795843209. Curr Med Chem. 2011. PMID: 21568919 Review.
Cited by
-
An Amazing 30-Year Journey around the DABO Family: A Medicinal Chemistry Lesson on a Versatile Class of Non-nucleoside HIV-1 Reverse Transcriptase Inhibitors.J Med Chem. 2025 Mar 27;68(6):5993-6026. doi: 10.1021/acs.jmedchem.4c02848. Epub 2025 Mar 7. J Med Chem. 2025. PMID: 40053382 Free PMC article. Review.
-
Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro.PLoS One. 2014 Aug 21;9(8):e105617. doi: 10.1371/journal.pone.0105617. eCollection 2014. PLoS One. 2014. PMID: 25144636 Free PMC article.
-
SJP-L-5 inhibits HIV-1 polypurine tract primed plus-strand DNA elongation, indicating viral DNA synthesis initiation at multiple sites under drug pressure.Sci Rep. 2018 Feb 7;8(1):2574. doi: 10.1038/s41598-018-20954-5. Sci Rep. 2018. PMID: 29416083 Free PMC article.
-
Recent Development of MOF-Based Photothermal Agent for Tumor Ablation.Front Chem. 2022 Mar 16;10:841316. doi: 10.3389/fchem.2022.841316. eCollection 2022. Front Chem. 2022. PMID: 35372266 Free PMC article. Review.
-
Structure-Based Discovery and Characterization of a Preclinical Drug Candidate for the Treatment of HIV-1 Infection.Viruses. 2022 Oct 28;14(11):2390. doi: 10.3390/v14112390. Viruses. 2022. PMID: 36366488 Free PMC article.
References
-
- UNAIDS (2012) World AIDS Day Report: Results. Geneva, Switzerland: UNAIDS. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiolo.... Accessed 6 August 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

