Population genetic structure of Streptococcus pneumoniae in Kilifi, Kenya, prior to the introduction of pneumococcal conjugate vaccine
- PMID: 24282605
- PMCID: PMC3839905
- DOI: 10.1371/journal.pone.0081539
Population genetic structure of Streptococcus pneumoniae in Kilifi, Kenya, prior to the introduction of pneumococcal conjugate vaccine
Abstract
Background: The 10-valent pneumococcal conjugate vaccine (PCV10) was introduced in Kenya in 2011. Introduction of any PCV will perturb the existing pneumococcal population structure, thus the aim was to genotype pneumococci collected in Kilifi before PCV10.
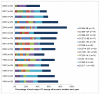
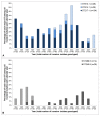
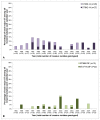
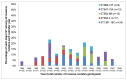
Methods and findings: Using multilocus sequence typing (MLST), we genotyped >1100 invasive and carriage pneumococci from children, the largest collection genotyped from a single resource-poor country and reported to date. Serotype 1 was the most common serotype causing invasive disease and was rarely detected in carriage; all serotype 1 isolates were members of clonal complex (CC) 217. There were temporal fluctuations in the major circulating sequence types (STs); and although 1-3 major serotype 1, 14 or 23F STs co-circulated annually, the two major serotype 5 STs mainly circulated independently. Major STs/CCs also included isolates of serotypes 3, 12F, 18C and 19A and each shared ≤ 2 MLST alleles with STs that circulate widely elsewhere. Major CCs associated with non-PCV10 serotypes were predominantly represented by carriage isolates, although serotype 19A and 12F CCs were largely invasive and a serotype 10A CC was equally represented by invasive and carriage isolates.
Conclusions: Understanding the pre-PCV10 population genetic structure in Kilifi will allow for the detection of changes in prevalence of the circulating genotypes and evidence for capsular switching post-vaccine implementation.
Conflict of interest statement
Figures
Similar articles
-
Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies.Lancet Glob Health. 2014 Jul;2(7):e397-405. doi: 10.1016/S2214-109X(14)70224-4. Epub 2014 May 28. Lancet Glob Health. 2014. PMID: 25103393 Free PMC article.
-
Serotypes and genotypes of invasive Streptococcus pneumoniae before and after PCV10 implementation in southern Brazil.PLoS One. 2014 Oct 30;9(10):e111129. doi: 10.1371/journal.pone.0111129. eCollection 2014. PLoS One. 2014. PMID: 25356595 Free PMC article.
-
Serotype and molecular diversity of nasopharyngeal Streptococcus pneumoniae isolates from children before and after vaccination with the ten-valent pneumococcal conjugate vaccine (PCV10) in Ethiopia.BMC Infect Dis. 2019 May 10;19(1):409. doi: 10.1186/s12879-019-4024-1. BMC Infect Dis. 2019. PMID: 31077141 Free PMC article.
-
CIRCULATING CLONAL COMPLEXES AND SEQUENCE TYPES OF STREPTOCOCCUS PNEUMONIAE SEROTYPE 19A WORLDWIDE: THE IMPORTANCE OF MULTIDRUG RESISTANCE: A SYSTEMATIC LITERATURE REVIEW.Expert Rev Vaccines. 2021 Jan;20(1):45-57. doi: 10.1080/14760584.2021.1873136. Epub 2021 Feb 17. Expert Rev Vaccines. 2021. PMID: 33507135
-
Streptococcus pneumoniae serotype 19A: worldwide epidemiology.Expert Rev Vaccines. 2017 Oct;16(10):1007-1027. doi: 10.1080/14760584.2017.1362339. Epub 2017 Aug 28. Expert Rev Vaccines. 2017. PMID: 28783380 Review.
Cited by
-
Characterisation of Invasive Streptococcus pneumoniae Isolated from Cambodian Children between 2007 - 2012.PLoS One. 2016 Jul 22;11(7):e0159358. doi: 10.1371/journal.pone.0159358. eCollection 2016. PLoS One. 2016. PMID: 27448096 Free PMC article.
-
Population snapshot of Streptococcus pneumoniae causing invasive disease in South Africa prior to introduction of pneumococcal conjugate vaccines.PLoS One. 2014 Sep 18;9(9):e107666. doi: 10.1371/journal.pone.0107666. eCollection 2014. PLoS One. 2014. PMID: 25233455 Free PMC article.
-
Genomic Analyses of >3,100 Nasopharyngeal Pneumococci Revealed Significant Differences Between Pneumococci Recovered in Four Different Geographical Regions.Front Microbiol. 2019 Feb 25;10:317. doi: 10.3389/fmicb.2019.00317. eCollection 2019. Front Microbiol. 2019. PMID: 30858837 Free PMC article.
-
Pre-vaccine serotype composition within a lineage signposts its serotype replacement - a carriage study over 7 years following pneumococcal conjugate vaccine use in the UK.Microb Genom. 2017 Jun 9;3(6):e000119. doi: 10.1099/mgen.0.000119. eCollection 2017 Jun 30. Microb Genom. 2017. PMID: 29026652 Free PMC article.
-
Molecular epidemiology of Streptococcus pneumoniae isolates causing invasive and noninvasive infection in Ethiopia.Sci Rep. 2024 Sep 13;14(1):21409. doi: 10.1038/s41598-024-72762-9. Sci Rep. 2024. PMID: 39271789 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) (2005) Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998-2003. MMWR Morb Mortal Wkly Rep 54: 893-897. PubMed: 16163262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

