Higher nucleoporin-Importinβ affinity at the nuclear basket increases nucleocytoplasmic import
- PMID: 24282617
- PMCID: PMC3840022
- DOI: 10.1371/journal.pone.0081741
Higher nucleoporin-Importinβ affinity at the nuclear basket increases nucleocytoplasmic import
Abstract
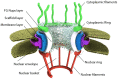
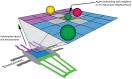
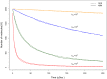
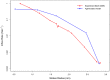
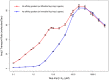
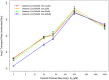
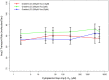
Several in vitro studies have shown the presence of an affinity gradient in nuclear pore complex proteins for the import receptor Importinβ, at least partially contributing to nucleocytoplasmic transport, while others have historically argued against the presence of such a gradient. Nonetheless, the existence of an affinity gradient has remained an uncharacterized contributing factor. To shed light on the affinity gradient theory and better characterize how the existence of such an affinity gradient between the nuclear pore and the import receptor may influence the nucleocytoplasmic traffic, we have developed a general-purpose agent based modeling (ABM) framework that features a new method for relating rate constants to molecular binding and unbinding probabilities, and used our ABM approach to quantify the effects of a wide range of forward and reverse nucleoporin-Importinβ affinity gradients. Our results indicate that transport through the nuclear pore complex is maximized with an effective macroscopic affinity gradient of 2000 µM, 200 µM and 10 µM in the cytoplasmic, central channel and nuclear basket respectively. The transport rate at this gradient is approximately 10% higher than the transport rate for a comparable pore lacking any affinity gradient, which has a peak transport rate when all nucleoporins have an affinity of 200 µM for Importinβ. Furthermore, this optimal ratio of affinity gradients is representative of the ratio of affinities reported for the yeast nuclear pore complex--suggesting that the affinity gradient seen in vitro is highly optimized.
Conflict of interest statement
Figures


References
-
- Loschberger A, van de Linde S, Dabauvalle MC, Rieger B, Heilemann M, et al. (2012) Super-resolution imaging visualizes the eightfold symmetry of gp210 proteins around the nuclear pore complex and resolves the central channel with nanometer resolution. J Cell Sci 125: 570–575. - PubMed
-
- Rout MP, Aitchison JD, Magnasco MO, Chait BT (2003) Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 13: 622–628. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

