ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome
- PMID: 24283224
- PMCID: PMC4727443
- DOI: 10.1056/NEJMoa1304603
ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome
Abstract
Background: Corticotropin-independent macronodular adrenal hyperplasia may be an incidental finding or it may be identified during evaluation for Cushing's syndrome. Reports of familial cases and the involvement of both adrenal glands suggest a genetic origin of this condition.
Methods: We genotyped blood and tumor DNA obtained from 33 patients with corticotropin-independent macronodular adrenal hyperplasia (12 men and 21 women who were 30 to 73 years of age), using single-nucleotide polymorphism arrays, microsatellite markers, and whole-genome and Sanger sequencing. The effects of armadillo repeat containing 5 (ARMC5) inactivation and overexpression were tested in cell-culture models.
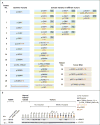
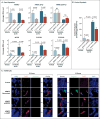
Results: The most frequent somatic chromosome alteration was loss of heterozygosity at 16p (in 8 of 33 patients for whom data were available [24%]). The most frequent mutation identified by means of whole-genome sequencing was in ARMC5, located at 16p11.2. ARMC5 mutations were detected in tumors obtained from 18 of 33 patients (55%). In all cases, both alleles of ARMC5 carried mutations: one germline and the other somatic. In 4 patients with a germline ARMC5 mutation, different nodules from the affected adrenals harbored different secondary ARMC5 alterations. Transcriptome-based classification of corticotropin-independent macronodular adrenal hyperplasia indicated that ARMC5 mutations influenced gene expression, since all cases with mutations clustered together. ARMC5 inactivation decreased steroidogenesis in vitro, and its overexpression altered cell survival.
Conclusions: Some cases of corticotropin-independent macronodular adrenal hyperplasia appear to be genetic, most often with inactivating mutations of ARMC5, a putative tumor-suppressor gene. Genetic testing for this condition, which often has a long and insidious prediagnostic course, might result in earlier identification and better management. (Funded by Agence Nationale de la Recherche and others.).
Figures

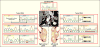
Comment in
-
Heredity and cortisol regulation in bilateral macronodular adrenal hyperplasia.N Engl J Med. 2013 Nov 28;369(22):2147-9. doi: 10.1056/NEJMe1312792. N Engl J Med. 2013. PMID: 24283229 No abstract available.
References
-
- Hsiao HP, Kirschner LS, Bourdeau I, et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94:2930–7. - PMC - PubMed
-
- Lacroix A. ACTH-independent macronodular adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23:245–59. - PubMed
-
- Swain JM, Grant CS, Schlinkert RT, et al. Corticotropin-independent macronodular adrenal hyperplasia: a clinicopathologic correlation. Arch Surg. 1998;133:541–5. - PubMed
-
- Reznik Y, Allali-Zerah V, Chayvialle JA, et al. Food-dependent Cushing’s syndrome mediated by aberrant adrenal sensitivity to gastric inhibitory polypeptide. N Engl J Med. 1992;327:981–6. - PubMed
-
- Libé R, Coste J, Guignat L, et al. Aberrant cortisol regulations in bilateral macronodular adrenal hyperplasia: a frequent finding in a prospective study of 32 patients with overt or subclinical Cushing’s syndrome. Eur J Endocrinol. 2010;163:129–38. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
