Flagellar central pair assembly in Chlamydomonas reinhardtii
- PMID: 24283352
- PMCID: PMC3895805
- DOI: 10.1186/2046-2530-2-15
Flagellar central pair assembly in Chlamydomonas reinhardtii
Abstract
Background: Most motile cilia and flagella have nine outer doublet and two central pair (CP) microtubules. Outer doublet microtubules are continuous with the triplet microtubules of the basal body, are templated by the basal body microtubules, and grow by addition of new subunits to their distal ("plus") ends. In contrast, CP microtubules are not continuous with basal body microtubules, raising the question of how these microtubules are assembled and how their polarity is established.
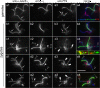
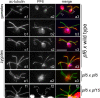
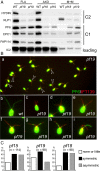
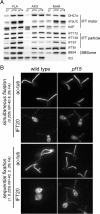
Methods: CP assembly in Chlamydomonas reinhardtii was analyzed by electron microscopy and wide-field and super-resolution immunofluorescence microscopy. To analyze CP assembly independently from flagellar assembly, the CP-deficient katanin mutants pf15 or pf19 were mated to wild-type cells. HA-tagged tubulin and the CP-specific protein hydin were used as markers to analyze de novo CP assembly inside the formerly mutant flagella.
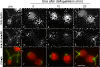
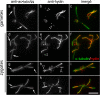
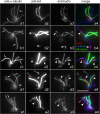
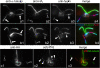
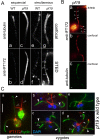
Results: In regenerating flagella, the CP and its projections assemble near the transition zone soon after the onset of outer doublet elongation. During de novo CP assembly in full-length flagella, the nascent CP was first apparent in a subdistal region of the flagellum. The developing CP replaces a fibrous core that fills the axonemal lumen of CP-deficient flagella. The fibrous core contains proteins normally associated with the C1 CP microtubule and proteins involved in intraflagellar transport (IFT). In flagella of the radial spoke-deficient mutant pf14, two pairs of CPs are frequently present with identical correct polarities.
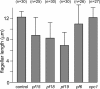
Conclusions: The temporal separation of flagellar and CP assembly in dikaryons formed by mating CP-deficient gametes to wild-type gametes revealed that the formation of the CP does not require proximity to the basal body or transition zone, or to the flagellar tip. The observations on pf14 provide further support that the CP self-assembles without a template and eliminate the possibility that CP polarity is established by interaction with axonemal radial spokes. Polarity of the developing CP may be determined by the proximal-to-distal gradient of precursor molecules. IFT proteins accumulate in flagella of CP mutants; the abnormal distribution of IFT proteins may explain why these flagella are often shorter than normal.
Figures


References
-
- Mitchell DR, Smith B. Analysis of the central pair microtubule complex in Chlamydomonas reinhardtii. Methods Cell Biol. 2009;2:197–213. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

