MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer
- PMID: 24283360
- PMCID: PMC4317817
- DOI: 10.1111/cas.12329
MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer
Abstract
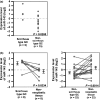
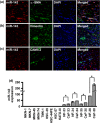
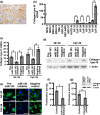
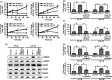
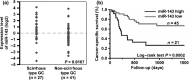
Gastric cancer (GC) is one of the most common malignancies worldwide. In particular, scirrhous type GC is highly metastatic and is characterized clinically by rapid disease progression and poor prognosis. MicroRNAs (miRNAs) play crucial roles in cancer development and progression. In the present study, we identified several miRNAs that are expressed at higher levels in scirrhous type GC than in non-scirrhous type GC by miRNA microarray analysis. Among these, microRNA-143 (miR-143) expression was higher in scirrhous type GC than in non-scirrhous types of GC. In situ hybridization and quantitative RT-PCR analysis showed that miR-143 is expressed by stromal fibroblasts but not by cancer cells. In stromal cells, miR-143 enhanced collagen type III expression in normal gastric fibroblasts and cancer-associated fibroblasts through activation of transforming growth factor-β)/SMAD signaling. Furthermore, high miR-143 expression in GC was associated with worse cancer-specific mortality (P = 0.0141). Multivariate analysis revealed that miR-143 was an independent prognostic factor. Treatment of GC cell lines with 5-aza-2'-deoxycytidine restored the expression of miR-143, and precursor miR-143 caused the inhibition of cancer cell invasion. These data suggest that miR-143 regulates fibrosis of scirrhous type GC through induction of collagen expression in stromal fibroblasts and that miR-143 expression serves as a prognostic marker of GC.
Keywords: Collagen type III; fibroblast; gastric cancer; microRNA-143; transforming growth factor-β.
© 2013 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–15. - PubMed
-
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
-
- Otsuji E, Kuriu Y, Okamoto K, et al. Outcome of surgical treatment for patients with scirrhous carcinoma of the stomach. Am J Surg. 2004;188:327–32. - PubMed
-
- Ikeguchi M, Miyake T, Matsunaga T, et al. Recent results of therapy for scirrhous gastric cancer. Surg Today. 2009;39:290–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

