High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells
- PMID: 24283362
- PMCID: PMC3929122
- DOI: 10.1089/dna.2013.2161
High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells
Abstract
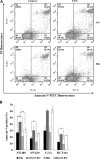
5-Fluorouracil (5-FU)-based chemotherapy is widely used for the treatment of colorectal cancer (CRC). While optimal doses of 5-FU are generally established based on a patient's estimated body surface area, the plasma concentrations of 5-FU vary among patients. In addition, hyperglycemia in patients with CRC has been reported as a risk factor in poor prognosis. The aim of the present study was to investigate whether hyperglycemia affects antiproliferative effect of 5-FU on the human colon cancer cells (SW480, SW620, LoVo, and HCT116). Growth inhibition of 5-FU was accessed by WST-8 assay. The effect of high glucose (HG, 15 mM) and 5-FU on the cellular proliferation was evaluated by flow cytometry analysis using 5-ethynyl-2'-deoxy-uridine (EdU) incorporation plus 7-AAD. Cell death was determined by flow cytometry using Annexin V-FITC and PI. The results showed that HG, compared to physiological normal glucose (NG) concentration (5 mM), leads to increased cell proliferation and increased GI50 of 5-FU in the four colon cancer cell lines. When the cells were pretreated with a low-dose 5-FU in NG condition, subsequent HG treatment eliminated inhibitory effect of 5-FU in cancer cell growth. In the presence of 5-FU (0.5 μg/mL for LoVo and HCT116; 1 μg/mL for SW480 and SW620), culture with HG for 72 h does not significantly altered cell cycle profile in the four cell lines but significantly increased DNA replication in SW620 (21%) and LoVo (17%). Flow cytometric analysis showed that HG protects cells against 5-FU-induced cell death in SW480. Finally, HG did not alter intracellular level of reactive oxygen species (ROS), although 5-FU indeed induced higher intracellular level of ROS. In conclusion, HG attenuates growth inhibition of 5-FU and our results indicate that decreased cell death and increased DNA replication may account for the attenuating effect of a HG environment on 5-FU-induced tumor growth inhibition.
Figures

 ) for 24 h. Then, the cells were treated with various concentration of 5-fluorouracil (5-FU) for 3 days. The cellular proliferation was assessed by WST-8 assay as described. Cellular proliferation of each colon cancer cell was significantly enhanced by the treatment of high glucose. The proliferation diminished with the increase of 5-FU dose. High glucose eliminated the growth inhibition of 5-FU when the dose of 5-FU was at least higher than GI50 for each cells. These analyses were mean±standard deviation (SD) of three independent experiments from at least three replications. **p<0.01, *p<0.05.
) for 24 h. Then, the cells were treated with various concentration of 5-fluorouracil (5-FU) for 3 days. The cellular proliferation was assessed by WST-8 assay as described. Cellular proliferation of each colon cancer cell was significantly enhanced by the treatment of high glucose. The proliferation diminished with the increase of 5-FU dose. High glucose eliminated the growth inhibition of 5-FU when the dose of 5-FU was at least higher than GI50 for each cells. These analyses were mean±standard deviation (SD) of three independent experiments from at least three replications. **p<0.01, *p<0.05.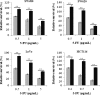

Similar articles
-
In vitro additive antitumor effects of dimethoxycurcumin and 5-fluorouracil in colon cancer cells.Cancer Med. 2017 Jul;6(7):1698-1706. doi: 10.1002/cam4.1114. Epub 2017 Jun 2. Cancer Med. 2017. PMID: 28573788 Free PMC article.
-
Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy.Cancer Res. 2001 Feb 1;61(3):1029-37. Cancer Res. 2001. PMID: 11221829
-
The mechanisms of 5-FU-PLA-O-CMC-NPS-mediated inhibition of the proliferation of colorectal cancer cell line SW480.Tumour Biol. 2014 Jun;35(6):6095-103. doi: 10.1007/s13277-014-1807-2. Epub 2014 Apr 17. Tumour Biol. 2014. PMID: 24740560
-
Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines.World J Gastroenterol. 2002 Oct;8(5):841-6. doi: 10.3748/wjg.v8.i5.841. World J Gastroenterol. 2002. PMID: 12378627 Free PMC article.
-
Enhanced Susceptibility to 5-Fluorouracil in Human Colon Cancer Cells by Silencing of GRP78.Anticancer Res. 2017 Jun;37(6):2975-2984. doi: 10.21873/anticanres.11651. Anticancer Res. 2017. PMID: 28551635
Cited by
-
Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications.J Hematol Oncol. 2022 Nov 1;15(1):160. doi: 10.1186/s13045-022-01358-5. J Hematol Oncol. 2022. PMID: 36319992 Free PMC article. Review.
-
High glucose dephosphorylates serine 46 and inhibits p53 apoptotic activity.J Exp Clin Cancer Res. 2014 Sep 27;33(1):79. doi: 10.1186/s13046-014-0079-4. J Exp Clin Cancer Res. 2014. PMID: 25260780 Free PMC article.
-
Hyperglycemia triggers HIPK2 protein degradation.Oncotarget. 2017 Jan 3;8(1):1190-1203. doi: 10.18632/oncotarget.13595. Oncotarget. 2017. PMID: 27901482 Free PMC article.
-
Diabetes and Colorectal Cancer Risk: A New Look at Molecular Mechanisms and Potential Role of Novel Antidiabetic Agents.Int J Mol Sci. 2021 Nov 17;22(22):12409. doi: 10.3390/ijms222212409. Int J Mol Sci. 2021. PMID: 34830295 Free PMC article. Review.
-
Effects of hyperglycemia on the progression of tumor diseases.J Exp Clin Cancer Res. 2019 Jul 23;38(1):327. doi: 10.1186/s13046-019-1309-6. J Exp Clin Cancer Res. 2019. PMID: 31337431 Free PMC article. Review.
References
-
- Barnes B.R., and Zierath J.R. (2005). Role of AMP-activated protein kinase in the control of glucose homeostasis. Curr Mol Med 5,341–348 - PubMed
-
- Buck S.B., Bradford J., Gee K.R., Agnew B.J., Clarke S.T., and Salic A. (2008). Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. Biotechniques 44,927–929 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

