β-catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation
- PMID: 24284205
- PMCID: PMC3865756
- DOI: 10.1242/dev.101550
β-catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation
Abstract
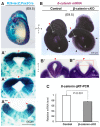
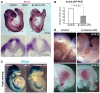
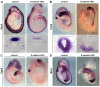
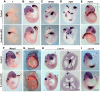
Non-canonical Wnt/planar cell polarity (PCP) signaling plays a primary role in the convergent extension that drives neural tube closure and body axis elongation. PCP signaling gene mutations cause severe neural tube defects (NTDs). However, the role of canonical Wnt/β-catenin signaling in neural tube closure and NTDs remains poorly understood. This study shows that conditional gene targeting of β-catenin in the dorsal neural folds of mouse embryos represses the expression of the homeobox-containing genes Pax3 and Cdx2 at the dorsal posterior neuropore (PNP), and subsequently diminishes the expression of the Wnt/β-catenin signaling target genes T, Tbx6 and Fgf8 at the tail bud, leading to spina bifida aperta, caudal axis bending and tail truncation. We demonstrate that Pax3 and Cdx2 are novel downstream targets of Wnt/β-catenin signaling. Transgenic activation of Pax3 cDNA can rescue the closure defect in the β-catenin mutants, suggesting that Pax3 is a key downstream effector of β-catenin signaling in the PNP closure process. Cdx2 is known to be crucial in posterior axis elongation and in neural tube closure. We found that Cdx2 expression is also repressed in the dorsal PNPs of Pax3-null embryos. However, the ectopically activated Pax3 in the β-catenin mutants cannot restore Cdx2 mRNA in the dorsal PNP, suggesting that the presence of both β-catenin and Pax3 is required for regional Cdx2 expression. Thus, β-catenin signaling is required for caudal neural tube closure and elongation, acting through the transcriptional regulation of key target genes in the PNP.
Keywords: Posterior neuropore (PNP); Spina bifida; Wnt/β-catenin signaling.
Figures

References
-
- Bassuk A. G., Kibar Z. (2009). Genetic basis of neural tube defects. Semin. Pediatr. Neurol. 16, 101–110 - PubMed
-
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 - PubMed
-
- Cadigan K. M. (2012). TCFs and Wnt/β-catenin signaling: more than one way to throw the switch. Curr. Top. Dev. Biol. 98, 1–34 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

