FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney
- PMID: 24284212
- PMCID: PMC3865747
- DOI: 10.1242/dev.089078
FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney
Abstract
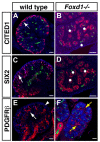
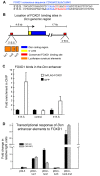
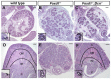
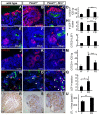
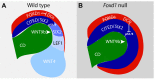
Forkhead transcription factors are essential for diverse processes in early embryonic development and organogenesis. Foxd1 is required during kidney development and its inactivation results in failure of nephron progenitor cell differentiation. Foxd1 is expressed in interstitial cells adjacent to nephron progenitor cells, suggesting an essential role for the progenitor cell niche in nephrogenesis. To better understand how cortical interstitial cells in general, and FOXD1 in particular, influence the progenitor cell niche, we examined the differentiation states of two progenitor cell subtypes in Foxd1(-/-) tissue. We found that although nephron progenitor cells are retained in a primitive CITED1-expressing compartment, cortical interstitial cells prematurely differentiate. To identify pathways regulated by FOXD1, we screened for target genes by comparison of Foxd1 null and wild-type tissues. We found that the gene encoding the small leucine-rich proteoglycan decorin (DCN) is repressed by FOXD1 in cortical interstitial cells, and we show that compound genetic inactivation of Dcn partially rescues the failure of progenitor cell differentiation in the Foxd1 null. We demonstrate that DCN antagonizes BMP/SMAD signaling, which is required for the transition of CITED1-expressing nephron progenitor cells to a state that is primed for WNT-induced epithelial differentiation. On the basis of these studies, we propose a mechanism for progenitor cell retention in the Foxd1 null in which misexpressed DCN produced by prematurely differentiated interstitial cells accumulates in the extracellular matrix, inhibiting BMP7-mediated transition of nephron progenitor cells to a compartment in which they can respond to epithelial induction signals.
Keywords: Interstitial cell; Kidney development; Mouse; Organogenesis; Progenitor niche.
Figures



Comment in
-
Stem cells: Decorin has role in differentiation.Nat Rev Nephrol. 2014 Feb;10(2):65. doi: 10.1038/nrneph.2013.271. Epub 2013 Dec 17. Nat Rev Nephrol. 2014. PMID: 24342960 No abstract available.
References
-
- Alexi X., Berditchevski F., Odintsova E. (2011). The effect of cell-ECM adhesion on signalling via the ErbB family of growth factor receptors. Biochem. Soc. Trans. 39, 568–573 - PubMed
-
- Ang S. L., Rossant J. (1994). HNF-3 beta is essential for node and notochord formation in mouse development. Cell 78, 561–574 - PubMed
-
- Barak H., Boyle S. C. (2011). Organ culture and immunostaining of mouse embryonic kidneys. Cold Spring Harb. Protoc. 2011, pdb prot5558 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

