Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience
- PMID: 24284214
- PMCID: PMC3886754
- DOI: 10.1161/JAHA.113.000424
Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience
Abstract
Background: The technique and safety of transcatheter patent ductus arteriosus (PDA) closure have evolved during the past 20 years. We sought to report a multicenter experience of PDA closure with a focus on the rate of adverse events (AE) and a review of institutional practice differences.
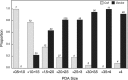
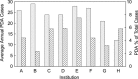
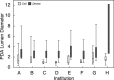
Methods and results: Outcome data on transcatheter PDA closure were collected at 8 centers prospectively using a multicenter registry (Congenital Cardiac Catheterization Project on Outcome Registry). Between February 2007 and June 2010, 496 PDA closures were recorded using a device in 338 (68%) or coils in 158 (32%). Most patients had an isolated PDA (90%). Fifty percent of patients were between 6 months and 3 years old, with only 40 patients (8%) <6 months old. Median minimum PDA diameter was 2.5 mm (range 1 to 12 mm; IQR 2 to 3 mm) for device closure and 1 mm (range 0.5 to 6 mm; IQR 1 to 2 mm) for coil closure (P<0.001). A device rather than coil was used in patients <3 years, weight <11 kg, and with a PDA minimum diameter >2 mm (all P<0.001). Three of 8 centers exclusively used a device for PDAs with a diameter >1.5 mm. In 9% of cases (n=46), an AE occurred; however, only 11 (2%) were classified as high severity. Younger age was associated with a higher AE rate. Coil-related AEs were more common than device-related AEs (10% versus 2%, P<0.001).
Conclusions: PDA closure in the present era has a very low rate of complications, although these are higher in younger children. Technical intervention-related events were more common in coil procedures compared with device procedures. For PDAs ≤2.5 mm in diameter, institutional differences in preference for device versus coil exist.
Keywords: PDA; adverse events; complications; interventional catheterization; safety.
Figures

References
-
- Faella HJ, Hijazi ZM. Closure of the patent ductus arteriosus with the Amplatzer PDA device: immediate results of the international clinical trial. Catheter Cardiovasc Interv. 2000; 51:50-54 - PubMed
-
- Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE. Multicenter USA Amplatzer patent ductus arteriosus occlusion device trail: initial and one‐year results. J Am Coll Cardiol. 2004; 44:513-519 - PubMed
-
- Bergersen L, Gauvreau K, Jenkins KJ, Lock JE. Adverse event rates in congenital cardiac catheterization: a new understanding of risks. Congenit Heart Dis. 2008; 3:90-105 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

