Peptide conjugation via CuAAC 'click' chemistry
- PMID: 24284482
- PMCID: PMC6270195
- DOI: 10.3390/molecules181113148
Peptide conjugation via CuAAC 'click' chemistry
Abstract
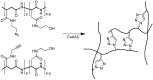
The copper (I)-catalyzed alkyne azide 1,3-dipolar cycloaddition (CuAAC) or 'click' reaction, is a highly versatile reaction that can be performed under a variety of reaction conditions including various solvents, a wide pH and temperature range, and using different copper sources, with or without additional ligands or reducing agents. This reaction is highly selective and can be performed in the presence of other functional moieties. The flexibility and selectivity has resulted in growing interest in the application of CuAAC in various fields. In this review, we briefly describe the importance of the structural folding of peptides and proteins and how the 1,4-disubstituted triazole product of the CuAAC reaction is a suitable isoster for an amide bond. However the major focus of the review is the application of this reaction to produce peptide conjugates for tagging and targeting purpose, linkers for multifunctional biomacromolecules, and reporter ions for peptide and protein analysis.
Figures

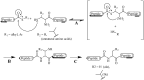



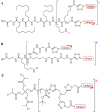
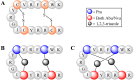
 ) represent hydrogen bonding sites. (PDB: 1HPV [59] and 1ZP8 [53]).
) represent hydrogen bonding sites. (PDB: 1HPV [59] and 1ZP8 [53]).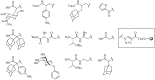
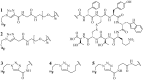


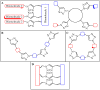







References
-
- Campbell N.A., Reece J.B. Biology. 6th ed. Pearson Education, Inc.; San Francisco, CA, USA: 2002. p. 1245.
-
- Curtius T. Ueber einige neue hippursaureanalog constituierte synthetisch dargestellte aminosauren. J. Prakt. Chem. 1882;26:145–208. doi: 10.1002/prac.18820260112. - DOI
-
- Fisher E., Fourneau E. Ueber einige derivate des glykokolls. Ber. Dtsch. Chem. Ges. 1901;34:2868–2879. doi: 10.1002/cber.190103402249. - DOI
-
- Merrifield R.B. Solid phase peptide synthesis.1. Synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963;85:2149–2154. doi: 10.1021/ja00897a025. - DOI
-
- Vigneaud V.D., Ressler C., Swan J.M., Roberts C.W., Katsoyannis P.G. The synthesis of oxytocin. J. Am. Chem. Soc. 1954;76:3115–3121.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

