Targeting Plasmodium PI(4)K to eliminate malaria
- PMID: 24284631
- PMCID: PMC3940870
- DOI: 10.1038/nature12782
Targeting Plasmodium PI(4)K to eliminate malaria
Abstract
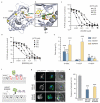
Achieving the goal of malaria elimination will depend on targeting Plasmodium pathways essential across all life stages. Here we identify a lipid kinase, phosphatidylinositol-4-OH kinase (PI(4)K), as the target of imidazopyrazines, a new antimalarial compound class that inhibits the intracellular development of multiple Plasmodium species at each stage of infection in the vertebrate host. Imidazopyrazines demonstrate potent preventive, therapeutic, and transmission-blocking activity in rodent malaria models, are active against blood-stage field isolates of the major human pathogens P. falciparum and P. vivax, and inhibit liver-stage hypnozoites in the simian parasite P. cynomolgi. We show that imidazopyrazines exert their effect through inhibitory interaction with the ATP-binding pocket of PI(4)K, altering the intracellular distribution of phosphatidylinositol-4-phosphate. Collectively, our data define PI(4)K as a key Plasmodium vulnerability, opening up new avenues of target-based discovery to identify drugs with an ideal activity profile for the prevention, treatment and elimination of malaria.
Figures



Comment in
-
Malaria: A step closer to elimination?Nat Rev Drug Discov. 2014 Jan;13(1):20. doi: 10.1038/nrd4216. Nat Rev Drug Discov. 2014. PMID: 24378798 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

