Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells
- PMID: 24284792
- PMCID: PMC3920002
- DOI: 10.1152/ajpcell.00381.2012
Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells
Abstract
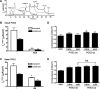
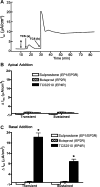
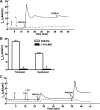
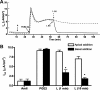
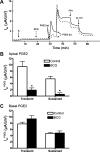
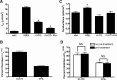
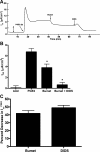
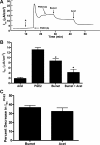
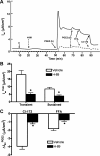
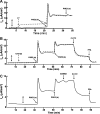
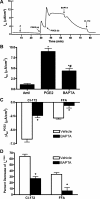
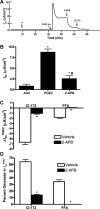
Under conditions of high dietary salt intake, prostaglandin E2 (PGE2) production is increased in the collecting duct and promotes urinary sodium chloride (NaCl) excretion; however, the molecular mechanisms by which PGE2 increases NaCl excretion in this context have not been clearly defined. We used the mouse inner medullary collecting duct (mIMCD)-K2 cell line to characterize mechanisms underlying PGE2-regulated NaCl transport. When epithelial Na(+) channels were inhibited, PGE2 exclusively stimulated basolateral EP4 receptors to increase short-circuit current (Isc(PGE2)). We found that Isc(PGE2) was sensitive to inhibition by H-89 and CFTR-172, indicating that EP4 receptors signal through protein kinase A to induce Cl(-) secretion via cystic fibrosis transmembrane conductance regulator (CFTR). Unexpectedly, we also found that Isc(PGE2) was sensitive to inhibition by BAPTA-AM (Ca(2+) chelator), 2-aminoethoxydiphenyl borate (2-APB) (inositol triphosphate receptor blocker), and flufenamic acid (FFA) [Ca(2+)-activated Cl(-) channel (CACC) inhibitor], suggesting that EP4 receptors also signal through Ca(2+) to induce Cl(-) secretion via CACC. Additionally, we observed that PGE2 stimulated an increase in Isc through crosstalk between cAMP and Ca(2+) signaling; BAPTA-AM or 2-APB inhibited a component of Isc(PGE2) that was sensitive to CFTR-172 inhibition; H-89 inhibited a component of Isc(PGE2) that was sensitive to FFA inhibition. Together, our findings indicate that PGE2 activates basolateral EP4 receptors and signals through both cAMP and Ca(2+) to stimulate Cl(-) secretion in IMCD-K2 cells. We propose that these signaling pathways, and the crosstalk between them, may provide a concerted mechanism for enhancing urinary NaCl excretion under conditions of high dietary NaCl intake.
Keywords: Ca2+-activated Cl- channel; EP4 receptor; collecting duct; cystic fibrosis transmembrane conductance regulator; prostaglandin E2.
Figures



Similar articles
-
Ca2+ and cAMP-activated Cl- conductances mediate Cl- secretion in a mouse renal inner medullary collecting duct cell line.J Physiol. 2000 Mar 1;523 Pt 2(Pt 2):325-38. doi: 10.1111/j.1469-7793.2000.t01-1-00325.x. J Physiol. 2000. PMID: 10699078 Free PMC article.
-
Activation of P2Y1 and P2Y2 receptors induces chloride secretion via calcium-activated chloride channels in kidney inner medullary collecting duct cells.Am J Physiol Renal Physiol. 2011 Sep;301(3):F544-53. doi: 10.1152/ajprenal.00709.2010. Epub 2011 Jun 8. Am J Physiol Renal Physiol. 2011. PMID: 21653634 Free PMC article.
-
Apical adenosine regulates basolateral Ca2+-activated potassium channels in human airway Calu-3 epithelial cells.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1443-53. doi: 10.1152/ajpcell.00556.2007. Epub 2008 Apr 2. Am J Physiol Cell Physiol. 2008. PMID: 18385283
-
Cystic fibrosis transmembrane conductance regulator (CFTR) and renal function.Wien Klin Wochenschr. 1997 Jun 27;109(12-13):457-64. Wien Klin Wochenschr. 1997. PMID: 9261986 Review.
-
Discovery and development of antisecretory drugs for treating diarrheal diseases.Clin Gastroenterol Hepatol. 2014 Feb;12(2):204-9. doi: 10.1016/j.cgh.2013.12.001. Epub 2013 Dec 4. Clin Gastroenterol Hepatol. 2014. PMID: 24316107 Free PMC article. Review.
Cited by
-
Phosphodiesterase Isoform Regulation of Cell Proliferation and Fluid Secretion in Autosomal Dominant Polycystic Kidney Disease.J Am Soc Nephrol. 2016 Apr;27(4):1124-34. doi: 10.1681/ASN.2015010047. Epub 2015 Aug 19. J Am Soc Nephrol. 2016. PMID: 26289612 Free PMC article.
-
New Insights into the Molecular Mechanisms Targeting Tubular Channels/Transporters in PKD Development.Kidney Dis (Basel). 2016 Oct;2(3):128-135. doi: 10.1159/000444839. Epub 2016 Mar 25. Kidney Dis (Basel). 2016. PMID: 27921040 Free PMC article. Review.
-
Evidence for cyclooxygenase-dependent sweating in young males during intermittent exercise in the heat.J Physiol. 2014 Dec 1;592(23):5327-39. doi: 10.1113/jphysiol.2014.280651. Epub 2014 Oct 17. J Physiol. 2014. PMID: 25326453 Free PMC article.
-
Renal Medullary Interstitial COX-2 (Cyclooxygenase-2) Is Essential in Preventing Salt-Sensitive Hypertension and Maintaining Renal Inner Medulla/Papilla Structural Integrity.Hypertension. 2018 Nov;72(5):1172-1179. doi: 10.1161/HYPERTENSIONAHA.118.11694. Hypertension. 2018. PMID: 30354807 Free PMC article.
-
Role of Serosal TRPV4-Constituted SOCE Mechanism in Secretagogues-Stimulated Intestinal Epithelial Anion Secretion.Front Pharmacol. 2021 Jul 14;12:684538. doi: 10.3389/fphar.2021.684538. eCollection 2021. Front Pharmacol. 2021. PMID: 34335254 Free PMC article.
References
-
- Andersson C, Servetnyk Z, Roomans GM. Activation of CFTR by genistein in human airway epithelial cell lines. Biochem Biophys Res Commun 308: 518–522, 2003 - PubMed
-
- Bao Y, Pucci ML, Chan BS, Lu R, Ito S, Schuster VL. Prostaglandin transporter PGT is expressed in cell types that synthesize and release prostanoids. Am J Physiol Renal Physiol 282: F1103–F1110, 2002 - PubMed
-
- Bito LZ. Inhibition of renal prostaglandin metabolism and excretion by probenecid, bromcresol green and indomethacin. Prostaglandins 12: 639–646, 1976 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

