Prostaglandins temporally regulate cytoplasmic actin bundle formation during Drosophila oogenesis
- PMID: 24284900
- PMCID: PMC3907279
- DOI: 10.1091/mbc.E13-07-0366
Prostaglandins temporally regulate cytoplasmic actin bundle formation during Drosophila oogenesis
Abstract
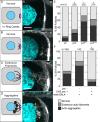
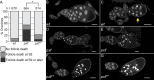
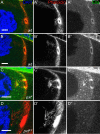
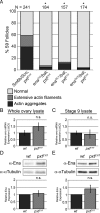
Prostaglandins (PGs)--lipid signals produced downstream of cyclooxygenase (COX) enzymes--regulate actin dynamics in cell culture and platelets, but their roles during development are largely unknown. Here we define a new role for Pxt, the Drosophila COX-like enzyme, in regulating the actin cytoskeleton--temporal restriction of actin remodeling during oogenesis. PGs are required for actin filament bundle formation during stage 10B (S10B). In addition, loss of Pxt results in extensive early actin remodeling, including actin filaments and aggregates, within the posterior nurse cells of S9 follicles; wild-type follicles exhibit similar structures at a low frequency. Hu li tai shao (Hts-RC) and Villin (Quail), an actin bundler, localize to all early actin structures, whereas Enabled (Ena), an actin elongation factor, preferentially localizes to those in pxt mutants. Reduced Ena levels strongly suppress early actin remodeling in pxt mutants. Furthermore, loss of Pxt results in reduced Ena localization to the sites of bundle formation during S10B. Together these data lead to a model in which PGs temporally regulate actin remodeling during Drosophila oogenesis by controlling Ena localization/activity, such that in S9, PG signaling inhibits, whereas at S10B, it promotes Ena-dependent actin remodeling.
Figures




Similar articles
-
Drosophila Fascin is a novel downstream target of prostaglandin signaling during actin remodeling.Mol Biol Cell. 2012 Dec;23(23):4567-78. doi: 10.1091/mbc.E12-05-0417. Epub 2012 Oct 10. Mol Biol Cell. 2012. PMID: 23051736 Free PMC article.
-
Pharmaco-Genetic Screen To Uncover Actin Regulators Targeted by Prostaglandins During Drosophila Oogenesis.G3 (Bethesda). 2019 Nov 5;9(11):3555-3565. doi: 10.1534/g3.119.400704. G3 (Bethesda). 2019. PMID: 31506320 Free PMC article.
-
Prostaglandins regulate invasive, collective border cell migration.Mol Biol Cell. 2020 Jul 15;31(15):1584-1594. doi: 10.1091/mbc.E19-10-0578. Epub 2020 May 20. Mol Biol Cell. 2020. PMID: 32432969 Free PMC article.
-
The Vast Utility of Drosophila Oogenesis.Methods Mol Biol. 2023;2626:1-36. doi: 10.1007/978-1-0716-2970-3_1. Methods Mol Biol. 2023. PMID: 36715897 Review.
-
Signaling between somatic follicle cells and the germline patterns the egg and embryo of Drosophila.Curr Top Dev Biol. 2020;140:55-86. doi: 10.1016/bs.ctdb.2019.10.004. Epub 2019 Nov 19. Curr Top Dev Biol. 2020. PMID: 32591083 Review.
Cited by
-
The lipid droplet protein Jabba promotes actin remodeling downstream of prostaglandin signaling during Drosophila oogenesis.bioRxiv [Preprint]. 2025 May 13:2025.05.09.653087. doi: 10.1101/2025.05.09.653087. bioRxiv. 2025. Update in: Mol Biol Cell. 2025 Sep 1;36(9):ar105. doi: 10.1091/mbc.E25-05-0218. PMID: 40463279 Free PMC article. Updated. Preprint.
-
A Diaphanous and Enabled-dependent asymmetric actin cable array repositions nuclei during Drosophila oogenesis.Development. 2022 Jul 1;149(13):dev197442. doi: 10.1242/dev.197442. Epub 2022 Jul 1. Development. 2022. PMID: 35686626 Free PMC article.
-
A drug repurposing screen reveals dopamine signaling as a critical pathway underlying potential therapeutics for the rare disease DPAGT1-CDG.PLoS Genet. 2024 Oct 28;20(10):e1011458. doi: 10.1371/journal.pgen.1011458. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39466823 Free PMC article.
-
Prostaglandins limit nuclear actin rod formation during Drosophila oogenesis.MicroPubl Biol. 2025 Apr 3;2025:10.17912/micropub.biology.001571. doi: 10.17912/micropub.biology.001571. eCollection 2025. MicroPubl Biol. 2025. PMID: 40255252 Free PMC article.
-
Modulation of Cell-Cell Interactions in Drosophila Oocyte Development.Cells. 2020 Jan 22;9(2):274. doi: 10.3390/cells9020274. Cells. 2020. PMID: 31979180 Free PMC article. Review.
References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004;11:36–42.
-
- Aszodi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S, Fassler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18:37–48. - PMC - PubMed
-
- Banan A, Smith GS, Kokoska ER, Miller TA. Role of actin cytoskeleton in prostaglandin-induced protection against ethanol in an intestinal epithelial cell line. J Surg Res. 2000;88:104–113. - PubMed
-
- Baum B, Li W, Perrimon N. A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr Biol. 2000;10:964–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

