Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding
- PMID: 24285115
- PMCID: PMC3920139
- DOI: 10.1152/ajpheart.00687.2013
Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding
Abstract
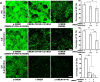
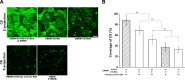
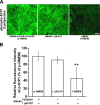
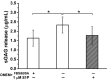
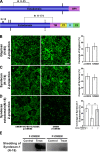
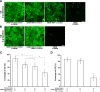
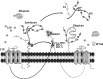
Endothelial cells (ECs) are covered by a surface glycocalyx layer that forms part of the barrier and mechanosensing functions of the blood-tissue interface. Removal of albumin in bathing media induces collapse or shedding of the glycocalyx. The electrostatic interaction between arginine residues on albumin, and negatively charged glycosaminoglycans (GAGs) in the glycocalyx have been hypothesized to stabilize the glycocalyx structure. Because albumin is one of the primary carriers of the phospholipid sphingosine-1-phosphate (S1P), we evaluated the alternate hypothesis that S1P, acting via S1P1 receptors, plays the primary role in stabilizing the endothelial glycocalyx. Using confocal microscopy on rat fat-pad ECs, we demonstrated that heparan sulfate (HS), chondroitin sulfate (CS), and ectodomain of syndecan-1 were shed from the endothelial cell surface after removal of plasma protein but were retained in the presence of S1P at concentrations of >100 nM. S1P1 receptor antagonism abolished the protection of the glycocalyx by S1P and plasma proteins. S1P reduced GAGs released after removal of plasma protein. The mechanism of protection from loss of glycocalyx components by S1P-dependent pathways was shown to be suppression of metalloproteinase (MMP) activity. General inhibition of MMPs protected against loss of CS and syndecan-1. Specific inhibition of MMP-9 and MMP-13 protected against CS loss. We conclude that S1P plays a critical role in protecting the glycocalyx via S1P1 and inhibits the protease activity-dependent shedding of CS, HS, and the syndecan-1 ectodomain. Our results provide new insight into the role for S1P in protecting the glycocalyx and maintaining vascular homeostasis.
Keywords: MMP; S1P; albumin; endothelial cells; glycocalyx.
Figures

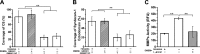
References
-
- Bode C, Sensken SC, Peest U, Beutel G, Thol F, Levkau B, Li Z, Bittman R, Huang T, Tolle M, van der Giet M, Graler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem 109: 1232–1243, 2010 - PubMed
-
- Brands J, Van Teeffelen JWGE, Van den Berg BM, Vink H. Role for glycocalyx perturbation in atherosclerosis development and associated microvascular dysfunction. Future Lipidol 2: 527–534, 2007
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

