Tunable visible and near-IR photoactivation of light-responsive compounds by using fluorophores as light-capturing antennas
- PMID: 24285381
- PMCID: PMC4036634
- DOI: 10.1002/anie.201308816
Tunable visible and near-IR photoactivation of light-responsive compounds by using fluorophores as light-capturing antennas
Abstract
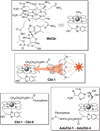
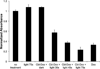
Although the corrin ring of vitamin B12 is unable to efficiently absorb light beyond 550 nm, it is shown that commercially available fluorophores can be used as antennas to capture long-wavelength light to promote scission of the Co-C bond at wavelengths up to 800 nm. The ability to control the molecular properties of bioactive species with long visible and near-IR light has implications for drug delivery, nanotechnology, and the spatiotemporal control of cellular behavior.
Keywords: cobalamins; drug delivery; energy transfer; fluorescence; photochemistry.
Copyright © 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
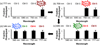



References
-
- Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A. Angew Chem Int Ed Engl. 2012;51:8446–8476. - PubMed
- Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Chem Rev. 2013;113:119–191. - PMC - PubMed
- Lee HM, Larson DR, Lawrence DS. ACS Chem Biol. 2009;4:409–427. - PMC - PubMed
-
- Goguen BN, Aemissegger A, Imperiali B. J Am Chem Soc. 2011;133:11038–11041. - PMC - PubMed
- Hagen V, Dekowski B, Nache V, Schmidt R, Geissler D, Lorenz D, Eichhorst J, Keller S, Kaneko H, Benndorf K, Wiesner B. Angew Chem Int Ed Engl. 2005;44:7887–7891. - PubMed
- Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H, Ellis-Davies GC. Nat Methods. 2010;7:123–125. - PMC - PubMed
- Menge C, Heckel A. Org Lett. 2011;13:4620–4623. - PubMed
- Priestman MA, Sun L, Lawrence DS. ACS Chem Biol. 2011;6:377–384. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

