Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation
- PMID: 24285499
- PMCID: PMC4073918
- DOI: 10.1152/ajprenal.00297.2013
Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation
Abstract
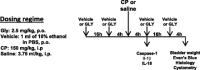
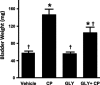
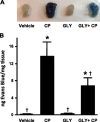
Bladder inflammation (cystitis) underlies numerous bladder pathologies and is elicited by a plethora of agents such as urinary tract infections, bladder outlet obstruction, chemotherapies, and catheters. Pattern recognition receptors [Toll-like receptors (TLRs) and Nod-like receptors (NLRs)] that recognize pathogen- and/or damage-associated molecular patterns (PAMPs and/or DAMPs, respectively) are key components of the innate immune system that coordinates the production (TLRs) and maturation (NLRs) of proinflammatory IL-1β. Despite multiple studies of TLRs in the bladder, none have investigated NLRs beyond one small survey. We now demonstrate that NLRP3 and NLRC4, and their binding partners apoptosis-associated speck-like protein containing a COOH-terminal caspase recruitment domain (ASC) and NLR family apoptosis inhibitory protein (NAIP), are expressed in the bladder and localized predominantly to the urothelia. Activated NLRs form inflammasomes that activate caspase-1. Placement of a NLRP3- or NLRC4-activating PAMP or NLRP3-activating DAMPs into the lumen of the bladder stimulated caspase-1 activity. To investigate inflammasomes in vivo, we induced cystitis with cyclophosphamide (CP, 150 mg/kg ip) in the presence or absence of the inflammasome inhibitor glyburide. Glyburide completely blocked CP-induced activation of caspase-1 and the production of IL-1β at 4 h. At 24 h, glyburide reduced two markers of inflammation by 30-50% and reversed much of the inflammatory morphology. Furthermore, glyburide reversed changes in bladder physiology (cystometry) induced by CP. In conclusion, NLRs/inflammasomes are present in the bladder urothelia and respond to DAMPs and PAMPs, whereas NLRP3 inhibition blocks bladder dysfunction in the CP model. The coordinated response of NLRs and TLRs in the urothelia represents a first-line innate defense that may provide an important target for pharmacological intervention.
Keywords: bladder; caspase-1; cystitis; inflammasome; inflammation.
Figures


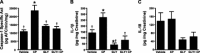


Similar articles
-
The NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) Inflammasome Mediates Inflammation and Voiding Dysfunction in a Lipopolysaccharide-Induced Rat Model of Cystitis.J Clin Cell Immunol. 2016 Feb;7(1):396. doi: 10.4172/2155-9899.1000396. Epub 2016 Feb 29. J Clin Cell Immunol. 2016. PMID: 27066297 Free PMC article.
-
Activation and regulation mechanisms of NOD-like receptors based on structural biology.Front Immunol. 2022 Sep 15;13:953530. doi: 10.3389/fimmu.2022.953530. eCollection 2022. Front Immunol. 2022. PMID: 36189327 Free PMC article. Review.
-
The potential repertoire of the innate immune system in the bladder: expression of pattern recognition receptors in the rat bladder and a rat urothelial cell line (MYP3 cells).Int Urol Nephrol. 2015 Dec;47(12):1953-64. doi: 10.1007/s11255-015-1126-6. Epub 2015 Oct 22. Int Urol Nephrol. 2015. PMID: 26490556 Free PMC article.
-
The NLRP3 Inflammasome Mediates Inflammation Produced by Bladder Outlet Obstruction.J Urol. 2016 May;195(5):1598-1605. doi: 10.1016/j.juro.2015.12.068. Epub 2015 Dec 18. J Urol. 2016. PMID: 26707508 Free PMC article.
-
NOD-Like Receptors in Infection, Immunity, and Diseases.Yonsei Med J. 2016 Jan;57(1):5-14. doi: 10.3349/ymj.2016.57.1.5. Yonsei Med J. 2016. PMID: 26632377 Free PMC article. Review.
Cited by
-
Diabetes causes NLRP3-dependent barrier dysfunction in mice with detrusor overactivity but not underactivity.Am J Physiol Renal Physiol. 2022 Dec 1;323(6):F616-F632. doi: 10.1152/ajprenal.00047.2022. Epub 2022 Sep 22. Am J Physiol Renal Physiol. 2022. PMID: 36135959 Free PMC article.
-
NLRP3 Promotes Diabetic Bladder Dysfunction and Changes in Symptom-Specific Bladder Innervation.Diabetes. 2019 Feb;68(2):430-440. doi: 10.2337/db18-0845. Epub 2018 Nov 13. Diabetes. 2019. PMID: 30425063 Free PMC article.
-
Elevated hydrostatic pressure stimulates ATP release which mediates activation of the NLRP3 inflammasome via P2X4 in rat urothelial cells.Int Urol Nephrol. 2018 Sep;50(9):1607-1617. doi: 10.1007/s11255-018-1948-0. Epub 2018 Aug 11. Int Urol Nephrol. 2018. PMID: 30099658 Free PMC article.
-
Why Are Some People with Lower Urinary Tract Symptoms (LUTS) Depressed? New Evidence That Peripheral Inflammation in the Bladder Causes Central Inflammation and Mood Disorders.Int J Mol Sci. 2023 Feb 1;24(3):2821. doi: 10.3390/ijms24032821. Int J Mol Sci. 2023. PMID: 36769140 Free PMC article. Review.
-
Extracorporeal shockwave against inflammation mediated by GPR120 receptor in cyclophosphamide-induced rat cystitis model.Mol Med. 2018 Nov 27;24(1):60. doi: 10.1186/s10020-018-0062-1. Mol Med. 2018. PMID: 30482157 Free PMC article.
References
-
- Auge C, Chene G, Dubourdeau M, Desoubzdanne D, Corman B, Palea S, Lluel P, Vergnolle N, Coelho AM. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol 707: 32–40, 2013 - PubMed
-
- Biondo C, Mancuso G, Beninati C, Iaria C, Romeo O, Cascio A, Teti G. The role of endosomal toll-like receptors in bacterial recognition. Eur Rev Med Pharmacol Sci 16: 1506–1512, 2012 - PubMed
-
- Bouchelouche K, Alvarez S, Andersen L, Nordling J, Horn T, Bouchelouche P. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J Urol 171: 462–466, 2004 - PubMed
-
- Bouchelouche K, Alvarez S, Horn T, Nordling J, Bouchelouche P. Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1beta and tumor necrosis factor-α. Urology 67: 214–219, 2006 - PubMed
-
- Bouchelouche K, Andresen L, Alvarez S, Nordling J, Nielsen OH, Bouchelouche P. Interleukin-4 and -13 induce the expression and release of monocyte chemoattractant protein 1, interleukin-6 and stem cell factor from human detrusor smooth muscle cells: synergy with interleukin-1β and tumor necrosis factor-α. J Urol 175: 760–765, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

