Nondividing, postpubertal rat sertoli cells resumed proliferation after transplantation
- PMID: 24285718
- PMCID: PMC4076399
- DOI: 10.1095/biolreprod.113.110197
Nondividing, postpubertal rat sertoli cells resumed proliferation after transplantation
Abstract
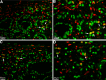
Conventionally, it was believed that Sertoli cells (SC) stopped proliferating at puberty and became terminally differentiated quiescent cells. However, recent studies have challenged that dogma. In this study, we transplanted nondividing SC isolated from 23- to 27-day-old postpubertal rats transduced with a recombinant adenoviral vector (containing furin-modified human proinsulin cDNA) into diabetic severe combined immunodeficiency mice. Immunostaining the grafts for cell proliferation markers, proliferating cell nuclear antigen (PCNA) and MKI67, revealed that transplanted SC within the grafts were proliferating. Possible causes for resumption of proliferation of SC could be viral transduction, cell isolation and culture, higher abdominal temperature at the transplant site, and/or transplantation. To test for these possible causes, double- immunofluorescence staining was performed for GATA4 (SC marker) and MKI67. None of the SC were positive for MKI67 in tissue collected during SC isolation and culture or at higher temperature. However, nontransduced SC stained positive for MKI67 after transplantation into rats, suggesting viral transduction was not a key factor for induction of SC proliferation. Interestingly, resumption in proliferative ability of nondividing SC was temporary, as SC stopped proliferating within 14 days of transplantation and did not proliferate thereafter. Quantification of 5-bromo-2'-deoxyuridine-labeled SC demonstrated that 7%-9% of the total transplanted SC were proliferating in the grafts. These data indicate for the first time that nondividing SC resumed proliferation after transplantation and further validate previous findings that SC are not terminally differentiated. Hence, transplantation of SC could provide a useful model with which to study the regulation of SC proliferation in vivo.
Keywords: Sertoli cells; proliferation; terminally differentiated; transplantation.
Figures
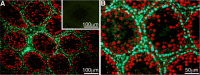
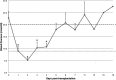

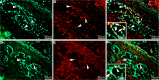
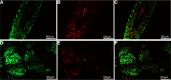

References
-
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 1998; 9: 411–416. - PubMed
-
- Mital P, Kaur G, Dufour JM. Immunoprotective Sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction 2010; 139: 495–504. - PubMed
-
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol 2011; 335: 60–68. - PubMed
-
- Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK. The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate. Biol Reprod 2005; 72: 1205–1217. - PubMed
-
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 1982; 203: 485–492. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

