Surrogacy assessment using principal stratification when surrogate and outcome measures are multivariate normal
- PMID: 24285772
- PMCID: PMC4023321
- DOI: 10.1093/biostatistics/kxt051
Surrogacy assessment using principal stratification when surrogate and outcome measures are multivariate normal
Abstract
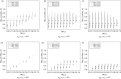
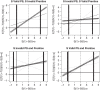
In clinical trials, a surrogate outcome variable (S) can be measured before the outcome of interest (T) and may provide early information regarding the treatment (Z) effect on T. Using the principal surrogacy framework introduced by Frangakis and Rubin (2002. Principal stratification in causal inference. Biometrics 58, 21-29), we consider an approach that has a causal interpretation and develop a Bayesian estimation strategy for surrogate validation when the joint distribution of potential surrogate and outcome measures is multivariate normal. From the joint conditional distribution of the potential outcomes of T, given the potential outcomes of S, we propose surrogacy validation measures from this model. As the model is not fully identifiable from the data, we propose some reasonable prior distributions and assumptions that can be placed on weakly identified parameters to aid in estimation. We explore the relationship between our surrogacy measures and the surrogacy measures proposed by Prentice (1989. Surrogate endpoints in clinical trials: definition and operational criteria. Statistics in Medicine 8, 431-440). The method is applied to data from a macular degeneration study and an ovarian cancer study.
Keywords: Bayesian estimation; Principal stratification; Surrogate endpoints.
Figures

Similar articles
-
Surrogacy assessment using principal stratification and a Gaussian copula model.Stat Methods Med Res. 2017 Feb;26(1):88-107. doi: 10.1177/0962280214539655. Epub 2016 Jul 11. Stat Methods Med Res. 2017. PMID: 24947559 Free PMC article.
-
Surrogacy assessment using principal stratification with multivariate normal and Gaussian copula models.Clin Trials. 2015 Aug;12(4):317-22. doi: 10.1177/1740774514561046. Epub 2014 Dec 9. Clin Trials. 2015. PMID: 25490988 Free PMC article.
-
A bayesian approach to surrogacy assessment using principal stratification in clinical trials.Biometrics. 2010 Jun;66(2):523-31. doi: 10.1111/j.1541-0420.2009.01303.x. Epub 2009 Aug 10. Biometrics. 2010. PMID: 19673864 Free PMC article.
-
Proportion of treatment effect explained: An overview of interpretations.Stat Methods Med Res. 2024 Jul;33(7):1278-1296. doi: 10.1177/09622802241259177. Epub 2024 Jul 25. Stat Methods Med Res. 2024. PMID: 39053571 Review.
-
Statistical challenges in the evaluation of surrogate endpoints in randomized trials.Control Clin Trials. 2002 Dec;23(6):607-25. doi: 10.1016/s0197-2456(02)00236-2. Control Clin Trials. 2002. PMID: 12505240 Review.
Cited by
-
A Bayesian Approach to Modeling Variance of Intensive Longitudinal Biomarker Data as a Predictor of Health Outcomes.Stat Med. 2024 Dec 30;43(30):5748-5764. doi: 10.1002/sim.10281. Epub 2024 Nov 14. Stat Med. 2024. PMID: 39542855 Free PMC article.
-
Evaluation of Surrogate Endpoints Using Information-Theoretic Measure of Association Based on Havrda and Charvat Entropy.Mathematics (Basel). 2022 Feb;10(3):465. doi: 10.3390/math10030465. Epub 2022 Jan 31. Mathematics (Basel). 2022. PMID: 35419255 Free PMC article.
-
A rank-based approach to evaluate a surrogate marker in a small sample setting.Biometrics. 2024 Jan 29;80(1):ujad035. doi: 10.1093/biomtc/ujad035. Biometrics. 2024. PMID: 38386359 Free PMC article.
-
A General Framework to Assess Complex Heterogeneity in the Strength of a Surrogate Marker.Stat Med. 2025 Feb 28;44(5):e70001. doi: 10.1002/sim.70001. Stat Med. 2025. PMID: 39915898 Free PMC article.
-
Links between causal effects and causal association for surrogacy evaluation in a gaussian setting.Stat Med. 2017 Nov 30;36(27):4243-4265. doi: 10.1002/sim.7430. Epub 2017 Aug 8. Stat Med. 2017. PMID: 28786131 Free PMC article.
References
-
- Barnard J., Mcculloch R., Meng X.-L. Modeling covariance matrices in terms of standard deviations and correlations, with applications to shrinkage. Statistica Sinica. 2000;10:1281–1311.
-
- Bartolucci F., Grilli L. Modeling partial compliance through copulas in a principal stratification framework. Journal of the American Statistical Association. 2011;106:469–479.
-
- Buyse M., Molenberghs G., Burzykowski T., Renard D., Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. - PubMed
-
- Chen H., Geng Z., Jia J. Criteria for surrogate endpoints. Journal of the Royal Statistical Society, Series B. 2007;69:919–932.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

