Auditory artificial grammar learning in macaque and marmoset monkeys
- PMID: 24285889
- PMCID: PMC3841451
- DOI: 10.1523/JNEUROSCI.2414-13.2013
Auditory artificial grammar learning in macaque and marmoset monkeys
Abstract
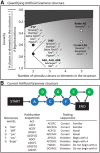
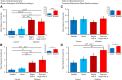
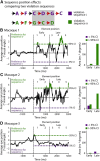
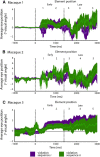
Artificial grammars (AG) are designed to emulate aspects of the structure of language, and AG learning (AGL) paradigms can be used to study the extent of nonhuman animals' structure-learning capabilities. However, different AG structures have been used with nonhuman animals and are difficult to compare across studies and species. We developed a simple quantitative parameter space, which we used to summarize previous nonhuman animal AGL results. This was used to highlight an under-studied AG with a forward-branching structure, designed to model certain aspects of the nondeterministic nature of word transitions in natural language and animal song. We tested whether two monkey species could learn aspects of this auditory AG. After habituating the monkeys to the AG, analysis of video recordings showed that common marmosets (New World monkeys) differentiated between well formed, correct testing sequences and those violating the AG structure based primarily on simple learning strategies. By comparison, Rhesus macaques (Old World monkeys) showed evidence for deeper levels of AGL. A novel eye-tracking approach confirmed this result in the macaques and demonstrated evidence for more complex AGL. This study provides evidence for a previously unknown level of AGL complexity in Old World monkeys that seems less evident in New World monkeys, which are more distant evolutionary relatives to humans. The findings allow for the development of both marmosets and macaques as neurobiological model systems to study different aspects of AGL at the neuronal level.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
