Long-term tolerability and maintenance of therapeutic response to sodium oxybate in an open-label extension study in patients with fibromyalgia
- PMID: 24286114
- PMCID: PMC3978755
- DOI: 10.1186/ar4375
Long-term tolerability and maintenance of therapeutic response to sodium oxybate in an open-label extension study in patients with fibromyalgia
Abstract
Introduction: The long-term safety and therapeutic response of sodium oxybate (SXB) in fibromyalgia syndrome (FM) patients were assessed for a combined period of up to 1 year in a prospective, multicenter, open-label, extension study in patients completing 1 of 2 phase 3 randomized, double-blind, controlled, 14-week trials that examined the efficacy and safety of SXB 4.5 g, SXB 6 g, and placebo for treatment of FM.
Methods: This extension study comprised an additional 38 weeks of treatment and was carried out at 130 clinical sites in 7 countries. Initial entry criteria for the previous 2 double-blind clinical trials required that patients aged ≥ 18 years met the American College of Rheumatology 1990 criteria for FM, had a body mass index (BMI) < 40 kg/m2, and had a score ≥ 50 on a 100-mm pain visual analog scale (VAS) at baseline. All patients began treatment in the extension study with SXB 4.5 g/night (administered in 2 equally divided doses) for at least 1 week, followed by possible serial 1.5 g/night dose increases to 9 g/night (maximum) or reductions to 4.5 g/night (minimum).
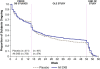
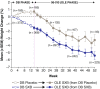
Results: Of the 560 FM patients enrolled in this extension study, 319 (57.0%) completed the study. The main reason for early discontinuation was adverse events (AEs; 23.0% of patients). Patients were primarily middle-aged (mean 46.9 ± 10.8 years), female (91.1%), white (91.4%), with a mean duration of FM symptoms of 9.9 ± 8.7 years. Serious AEs were experienced by 3.6% of patients. The most frequently reported AEs (incidence ≥ 5% at any dose or overall) were nausea, headache, dizziness, nasopharyngitis, vomiting, sinusitis, diarrhea, anxiety, insomnia, influenza, somnolence, upper respiratory tract infection, muscle spasms, urinary tract infection, and gastroenteritis viral. Maintenance of SXB therapeutic response was demonstrated with continued improvement from controlled-study baseline in pain VAS, Fibromyalgia Impact Questionnaire (FIQ) total scores, and other measures. Responder analyses showed that 68.8% of patients achieved ≥ 30% reduction in pain VAS and 69.7% achieved ≥ 30% reduction in FIQ total score at study endpoint.
Conclusions: The long-term safety profile of SXB in FM patients was similar to that in the previously reported controlled clinical trials. Improvement in pain and other FM clinical domains was maintained during long-term use.
Trial registration: ClinicalTrials.gov NCT00423605.
Figures


References
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;15:160–172. - PubMed
-
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;15:600–610. - PubMed
-
- Jain AK, Carruthers BM, van de Sande MI, Barron SR, Donaldson CCS, Dunne JV, Gingrich E, Heffez DS, Leung FY-K, Malone DG, Romano TJ, Russell IJ, Saul D, Seibel DG. Fibromyalgia syndrome: Canadian clinical working case definition, diagnostic, and treatment protocols—a consensus document. J Musculoskelet Pain. 2003;15:3–107.
-
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;15:19–28. - PubMed
-
- Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, Russell IJ, Yunus MB. Health status and disease severity in fibromyalgia. Results of a six-center longitudinal study. Arthritis Rheum. 1997;15:1571–1579. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

