Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the γ1 subunit
- PMID: 24286368
- PMCID: PMC3930416
- DOI: 10.1111/jcmm.12187
Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the γ1 subunit
Abstract
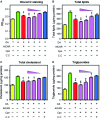
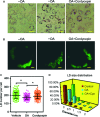
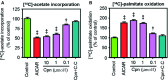
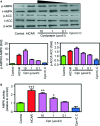
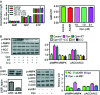
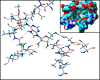
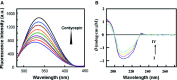
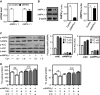
Cordycepin is a bioactive component of the fungus Cordyceps militaris. Previously, we showed that cordycepin can alleviate hyperlipidemia through enhancing the phosphorylation of AMP-activated protein kinase (AMPK), but the mechanism of this stimulation is unknown. Here, we investigated the potential mechanisms of cordycepin-induced AMPK activation in HepG2 cells. Treatment with cordycepin largely reduced oleic acid (OA)-elicited intracellular lipid accumulation and increased AMPK activity in a dose-dependent manner. Cordycepin-induced AMPK activation was not accompanied by changes in either the intracellular levels of AMP or the AMP/ATP ratio, nor was it influenced by calmodulin-dependent protein kinase kinase (CaMKK) inhibition; however, this activation was significantly suppressed by liver kinase B1 (LKB1) knockdown. Molecular docking, fluorescent and circular dichroism measurements showed that cordycepin interacted with the γ1 subunit of AMPK. Knockdown of AMPKγ1 by siRNA substantially abolished the effects of cordycepin on AMPK activation and lipid regulation. The modulating effects of cordycepin on the mRNA levels of key lipid regulatory genes were also largely reversed when AMPKγ1 expression was inhibited. Together, these data suggest that cordycepin may inhibit intracellular lipid accumulation through activation of AMPK via interaction with the γ1 subunit.
Keywords: AMPK; Cordycepin; LKB1; Molecular docking.
© 2013 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes.Am J Physiol Cell Physiol. 2007 Oct;293(4):C1395-403. doi: 10.1152/ajpcell.00115.2007. Epub 2007 Aug 8. Am J Physiol Cell Physiol. 2007. PMID: 17687000
-
Mechanism of Activation of AMPK by Cordycepin.Cell Chem Biol. 2020 Feb 20;27(2):214-222.e4. doi: 10.1016/j.chembiol.2020.01.004. Epub 2020 Jan 27. Cell Chem Biol. 2020. PMID: 31991096 Free PMC article.
-
eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1.Biochim Biophys Acta. 2011 Feb;1813(2):322-31. doi: 10.1016/j.bbamcr.2010.12.001. Epub 2010 Dec 9. Biochim Biophys Acta. 2011. PMID: 21145922
-
AMP-activated protein kinase in metabolic control and insulin signaling.Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review.
-
AMP-Activated Protein Kinase: Do We Need Activators or Inhibitors to Treat or Prevent Cancer?Int J Mol Sci. 2020 Dec 27;22(1):186. doi: 10.3390/ijms22010186. Int J Mol Sci. 2020. PMID: 33375416 Free PMC article. Review.
Cited by
-
The Prophylactic and Therapeutic Effects of Fermented Cordyceps sinensis Powder, Cs-C-Q80, on Subcortical Ischemic Vascular Dementia in Mice.Evid Based Complement Alternat Med. 2018 Dec 18;2018:4362715. doi: 10.1155/2018/4362715. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30662512 Free PMC article.
-
Cordycepin augments the chemosensitivity of osteosarcoma to cisplatin by activating AMPK and suppressing the AKT signaling pathway.Cancer Cell Int. 2021 Dec 25;21(1):706. doi: 10.1186/s12935-021-02411-y. Cancer Cell Int. 2021. PMID: 34953496 Free PMC article.
-
Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE-/- Mice.Mar Drugs. 2017 Nov 14;15(11):358. doi: 10.3390/md15110358. Mar Drugs. 2017. PMID: 29135917 Free PMC article.
-
The caffeic acid moiety plays an essential role in attenuating lipid accumulation by chlorogenic acid and its analogues.RSC Adv. 2019 Apr 17;9(22):12247-12254. doi: 10.1039/c8ra09395d. eCollection 2019 Apr 17. RSC Adv. 2019. PMID: 35515874 Free PMC article.
-
Fermented Cordyceps militaris Extract Prevents Hepatosteatosis and Adipocyte Hypertrophy in High Fat Diet-Fed Mice.Nutrients. 2019 May 6;11(5):1015. doi: 10.3390/nu11051015. Nutrients. 2019. PMID: 31064103 Free PMC article.
References
-
- Chen WL, Chen YL, Chiang YM, et al. Fenofibrate lowers lipid accumulation in myotubes by modulating the PPARalpha/AMPK/FoxO1/ATGL pathway. Biochem Pharmacol. 2012;84:522–31. - PubMed
-
- Lee SK, Lee JO, Kim JH, et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARalpha induction in 3T3-L1 preadipocytes. Cell Signal. 2012;24:2329–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

