A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011
- PMID: 24287460
- PMCID: PMC3854014
- DOI: 10.3390/ph6111335
A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011
Abstract
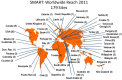
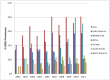
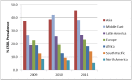
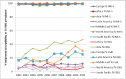
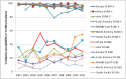
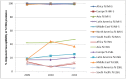
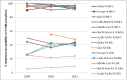
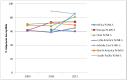
Surveillance of antimicrobial agent resistance provides important information to guide microbiologists and infectious disease specialists understanding of the control and the spread of resistance mechanisms within the local environment. Continued monitoring of antimicrobial resistance patterns in the community and in local hospital environments is essential to guide effective empiric therapy. The Study for Monitoring Antimicrobial Resistance Trends (SMART) has monitored the in vitro susceptibility patterns of clinical Gram-negative bacilli to antimicrobial agents collected worldwide from intra-abdominal infections since 2002 and urinary tract infections since 2009. Resistance trends, with a particular focus on carbapenem resistance and the rate of extended-spectrum β-lactamases (ESBLs), were analyzed. Isolates from intra-abdominal infections (n = 92,086) and urinary-tract infections (n = 24,705) were collected and tested using Clinical and Laboratory Standards Institute methods. This review presents carbapenem susceptibility and ESBL rates over ten years of SMART study analysis, including key publications during this period. The SMART study has proved to be a valuable resource in determining pathogen prevalence and antibiotic susceptibility over the last ten years and continues to provide evidence for regulatory susceptibility breakpoints and clinical decision making.
Figures

References
-
- Hawser S. Surveillance programmes and antibiotic resistance: Worldwide and regional monitoring of antibiotic resistance trends. Antibiotic Resist. 2012;211:31–43. - PubMed
-
- Felmingham D., White A.R., Jacobs M.R., Appelbaum P.C., Poupard J., Miller L.A., Grüneberg R.N. The Alexander Project: the benefits from a decade of surveillance. J. Antimicrob. Chemother. 2005;56:ii3–ii21. - PubMed
-
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standards—Ninth Edition. Clinical Laboratory Standards Institute; Wayne, PA, USA: 2012. (CLSI document M07-A9).
-
- Performance Standards for Antimicrobial Susceptibility Testing, Twenty-second Informational Supplement. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA, USA: 2013. (CLSI document M100-S23).
-
- Hawser S.P., Bouchillon S.K., Hoban D.J., Badal R.E. In vitro susceptibilities of aerobic and facultative anaerobic Gram-negative bacilli from patients with intra-abdominal infections worldwide from 2005–2007: Results from the SMART study. Int. J. Antimicrob. Agents. 2009;34:585–588. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

