Metabolic Interactions of Purine Derivatives with Human ABC Transporter ABCG2: Genetic Testing to Assess Gout Risk
- PMID: 24287461
- PMCID: PMC3854015
- DOI: 10.3390/ph6111347
Metabolic Interactions of Purine Derivatives with Human ABC Transporter ABCG2: Genetic Testing to Assess Gout Risk
Abstract
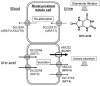
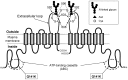
In mammals, excess purine nucleosides are removed from the body by breakdown in the liver and excretion from the kidneys. Uric acid is the end product of purine metabolism in humans. Two-thirds of uric acid in the human body is normally excreted through the kidney, whereas one-third undergoes uricolysis (decomposition of uric acid) in the gut. Elevated serum uric acid levels result in gout and could be a risk factor for cardiovascular disease and diabetes. Recent studies have shown that human ATP-binding cassette transporter ABCG2 plays a role of renal excretion of uric acid. Two non-synonymous single nucleotide polymorphisms (SNPs), i.e., 421C>A (major) and 376C>T (minor), in the ABCG2 gene result in impaired transport activity, owing to ubiquitination-mediated proteosomal degradation and truncation of ABCG2, respectively. These genetic polymorphisms are associated with hyperuricemia and gout. Allele frequencies of those SNPs are significantly higher in Asian populations than they are in African and Caucasian populations. A rapid and isothermal genotyping method has been developed to detect the SNP 421C>A, where one drop of peripheral blood is sufficient for the detection. Development of simple genotyping methods would serve to improve prevention and early therapeutic intervention for high-risk individuals in personalized healthcare.
Figures





References
-
- Giacomini K.M., Huang S.M., Tweedie D.J., Benet L.Z., Brouwer K.L., Chu X., Dahlin A., Evers R., Fischer V., Hillgren K.M., et al. Membrane transporters in drug development: Report from the FDA critical path initiative-sponsored workshop. Nat. Rev. Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. - DOI - PMC - PubMed
-
- Ishikawa T. Multidrug resistance: Genomics of ABC transporters. Nat. Encycl. Hum. Genome. 2003;4:154–160.
-
- Matsuo H., Takada T., Ichida K., Nakamura T., Nakayama A., Ikebuchi Y., Ito K., Kusanagi Y., Chiba T., Tadokoro S., et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout. A function-based genetic analysis in a Japanese population. Sci. Transl. Med. 2009;1 doi: 10.1126/scitranslmed.3000237. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

