Histone acetylation and arachidonic acid cytotoxicity in HepG2 cells overexpressing CYP2E1
- PMID: 24287576
- PMCID: PMC3927057
- DOI: 10.1007/s00210-013-0942-4
Histone acetylation and arachidonic acid cytotoxicity in HepG2 cells overexpressing CYP2E1
Abstract
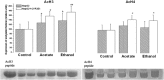
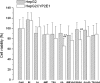
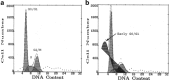
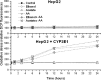
The aim of this work was to assess the role of ethanol-derived acetate and acetate-mediated histone acetylation in arachidonic acid-induced stress in HepG2 cells and cells overexpressing CYP2E1. Cells were grown for 7 days with 1 mM sodium acetate or 100 mM ethanol; their acetylated histone proteins and histone deacetylase 2 expression was quantified using Western blot. Ethanol- or acetate-pretreated cells were also treated for 24 h with 60 μM arachidonic acid to induce oxidative stress. Cytotoxicity was estimated by lactate dehydrogenase release, 3-[4,5-dimethylthiazolyl-2] 2,5-diphenyltetrazolium bromide test, and by DNA damage, while oxidative stress was quantified using dichlorofluorescein diacetate. Cells grown with ethanol or acetate had increased acetylated histone H3 levels in both cell types and elevated acetylated histone H4 levels in cells overexpressing CYP2E1 but not in naïve cells. In cells overexpressing CYP2E1 grown with ethanol, expression of histone deacetylase 2 was reduced by about 40 %. Arachidonic acid altered cell proliferation and was cytotoxic mostly to cells engineered to overexpress CYP2E1 but both effects were significantly lower in cells pretreated with ethanol or acetate. Cytotoxicity was also significantly decreased by 4-methylpyrazole--a CYP2E1 inhibitor and by trichostatin--an inhibitor of histone deacetylases. In cells pretreated with acetate or ethanol, the oxidative stress induced by arachidonic acid was also significantly lower. Our data indicate that histone hyperacetylation may in some extent protect the cells against oxidative stress. It is possible that acetate may act as an antioxidant at histone level. This mechanism may be relevant to alcohol-induced liver injury.
Figures



Similar articles
-
Ethanol metabolism by alcohol dehydrogenase or cytochrome P450 2E1 differentially impairs hepatic protein trafficking and growth hormone signaling.Am J Physiol Gastrointest Liver Physiol. 2017 Dec 1;313(6):G558-G569. doi: 10.1152/ajpgi.00027.2017. Epub 2017 Sep 1. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28864499 Free PMC article.
-
Inhibition of autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via elevated oxidative stress, mitochondria dysfunction and activation of p38 and JNK MAPK.Redox Biol. 2013 Nov 5;1(1):552-65. doi: 10.1016/j.redox.2013.10.008. eCollection 2013. Redox Biol. 2013. PMID: 24273738 Free PMC article.
-
Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo.Free Radic Biol Med. 2013 Oct;63:175-86. doi: 10.1016/j.freeradbiomed.2013.05.009. Epub 2013 May 10. Free Radic Biol Med. 2013. PMID: 23669278 Free PMC article.
-
CYP2E1-dependent toxicity and oxidative stress in HepG2 cells.Free Radic Biol Med. 2001 Dec 15;31(12):1539-43. doi: 10.1016/s0891-5849(01)00743-2. Free Radic Biol Med. 2001. PMID: 11744327 Review.
-
Oxidative stress mediated toxicity exerted by ethanol-inducible CYP2E1.Toxicol Appl Pharmacol. 2005 Sep 1;207(2 Suppl):70-6. doi: 10.1016/j.taap.2005.01.057. Toxicol Appl Pharmacol. 2005. PMID: 16019049 Review.
Cited by
-
Cytotoxicity and Oxidative Stress Induction by Root Canal Sealers in Human Periodontal Ligament Fibroblasts: An in vitro Study.Iran Endod J. 2021 Summer;16(3):164-175. doi: 10.22037/iej.v16i3.29449. Iran Endod J. 2021. PMID: 36704398 Free PMC article.
-
In silico Identification and Mechanism Exploration of Hepatotoxic Ingredients in Traditional Chinese Medicine.Front Pharmacol. 2019 May 3;10:458. doi: 10.3389/fphar.2019.00458. eCollection 2019. Front Pharmacol. 2019. PMID: 31130860 Free PMC article.
-
Oxidative products from alcohol metabolism differentially modulate pro-inflammatory cytokine expression in Kupffer cells and hepatocytes.Cytokine. 2016 Sep;85:109-19. doi: 10.1016/j.cyto.2016.06.014. Epub 2016 Jun 14. Cytokine. 2016. PMID: 27314544 Free PMC article.
-
Metformin and Probiotics Interplay in Amelioration of Ethanol-Induced Oxidative Stress and Inflammatory Response in an In Vitro and In Vivo Model of Hepatic Injury.Mediators Inflamm. 2021 Apr 15;2021:6636152. doi: 10.1155/2021/6636152. eCollection 2021. Mediators Inflamm. 2021. PMID: 33953643 Free PMC article.
References
-
- Buurman R, Gürlevik E, Schäffer V, Eilers M, Sandbothe M, Kreipe H, Wilken L, Schlegelberger B, Kühnel F, Skawran B. Histone deacetylases activate hepatocyte growth factor signaling by repressing microRNA-449 in hepatocellular carcinoma cells. Gastroenterology. 2012;143:811–820. doi: 10.1053/j.gastro.2012.05.033. - DOI - PubMed
-
- Carlisi D, Vassallo B, Lauricella M, Emanuele S, D’Anneo A, Di Leonardo E, Di Fazio P, Vento R, Tesoriere G. Histone deacetylase inhibitors induce in human hepatoma HepG2 cells acetylation of p53 and histones in correlation with apoptotic effects. Int J Oncol. 2008;32:177–184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

