Shellfish toxins targeting voltage-gated sodium channels
- PMID: 24287955
- PMCID: PMC3877881
- DOI: 10.3390/md11124698
Shellfish toxins targeting voltage-gated sodium channels
Abstract
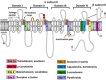
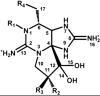
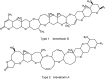
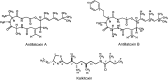
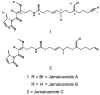
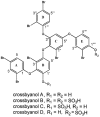
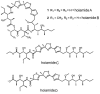
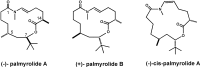
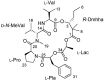
Voltage-gated sodium channels (VGSCs) play a central role in the generation and propagation of action potentials in excitable neurons and other cells and are targeted by commonly used local anesthetics, antiarrhythmics, and anticonvulsants. They are also common targets of neurotoxins including shellfish toxins. Shellfish toxins are a variety of toxic secondary metabolites produced by prokaryotic cyanobacteria and eukaryotic dinoflagellates in both marine and fresh water systems, which can accumulate in marine animals via the food chain. Consumption of shellfish toxin-contaminated seafood may result in potentially fatal human shellfish poisoning. This article provides an overview of the structure, bioactivity, and pharmacology of shellfish toxins that act on VGSCs, along with a brief discussion on their pharmaceutical potential for pain management.
Figures
References
-
- Cestèle S., Catterall W.A. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. - PubMed
-
- Isom L.L., DeJongh K.S., Patton D.E., Reber B.F., Offord X.J., Charbonneau H., Walsh K., Goldin A.L., Catterall W.A. Primary structure and functional expression of the β1 Subunit of the rat brain sodium channel. Science. 1992;256:839–842. - PubMed
-
- Isom L.L., Ragsdale D.S., De Jongh K.S., Westenbroek R.E., Reber B.F., Scheuer X.T., Catterall W.A. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. - PubMed
-
- Morgan K., Stevens E.B., Shah B., Cox P.J., Dixon A.K., Lee K., Pinnock R.D., Hughes J., Richardson P.J., Mizuguchi K., Jackson A.P. β3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. USA. 2000;97:2308–2313. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

