Toxic c17-sphinganine analogue mycotoxin, contaminating tunisian mussels, causes flaccid paralysis in rodents
- PMID: 24287956
- PMCID: PMC3877882
- DOI: 10.3390/md11124724
Toxic c17-sphinganine analogue mycotoxin, contaminating tunisian mussels, causes flaccid paralysis in rodents
Abstract
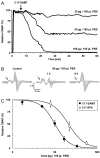
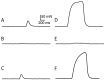
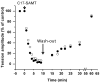
Severe toxicity was detected in mussels from Bizerte Lagoon (Northern Tunisia) using routine mouse bioassays for detecting diarrheic and paralytic toxins not associated to classical phytoplankton blooming. The atypical toxicity was characterized by rapid mouse death. The aim of the present work was to understand the basis of such toxicity. Bioassay-guided chromatographic separation and mass spectrometry were used to detect and characterize the fraction responsible for mussels' toxicity. Only a C17-sphinganine analog mycotoxin (C17-SAMT), with a molecular mass of 287.289 Da, was found in contaminated shellfish. The doses of C17-SAMT that were lethal to 50% of mice were 750 and 150 μg/kg following intraperitoneal and intracerebroventricular injections, respectively, and 900 μg/kg following oral administration. The macroscopic general aspect of cultures and the morphological characteristics of the strains isolated from mussels revealed that the toxicity episodes were associated to the presence of marine microfungi (Fusarium sp., Aspergillus sp. and Trichoderma sp.) in contaminated samples. The major in vivo effect of C17-SAMT on the mouse neuromuscular system was a dose- and time-dependent decrease of compound muscle action potential amplitude and an increased excitability threshold. In vitro, C17-SAMT caused a dose- and time-dependent block of directly- and indirectly-elicited isometric contraction of isolated mouse hemidiaphragms.
Figures



References
-
- Smith J.L., Boyer G.L., Zimba P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture. 2008;280:5–20. doi: 10.1016/j.aquaculture.2008.05.007. - DOI
-
- Zaccaroni A., Scaravelli D. Algal Toxins-Nature Occurrence and Detection. Springer Netherlands; Heidelberg, The Netherlands: 2008. Toxicity of Sea Algal Toxins to Human and Animal; pp. 91–158.
-
- Krys S., Arnich N., Fremy J.M. Rencontres en Toxinologie, Toxines Émergentes: Nouveaux Risques. Lavoisier; Paris, France: 2007. Emergence de nouvelles toxicités d’origine marine: Vers une évolution significative des dispositifs de sécurisation des coquillages? pp. 117–122.
-
- Abbas H.K., Duke S.O., Shier W.T., Riley R.T., Kraus G.A. The chemistry and biological activities of the natural products AAL toxin and the fumonisins. Adv. Exp. Med. Biol. 1996;391:293–308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

