Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study
- PMID: 24288014
- PMCID: PMC4316845
- DOI: 10.1136/annrheumdis-2013-204016
Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study
Erratum in
-
Correction: Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study.Ann Rheum Dis. 2016 Jul;75(7):e46. doi: 10.1136/annrheumdis-2013-204016corr1. Ann Rheum Dis. 2016. PMID: 27288391 Free PMC article. No abstract available.
Abstract
Objectives: To investigate the possibility of discontinuing adalimumab (ADA) for 1 year without flaring (DAS28-erythrocyte sedimentation rate (ESR) ≥3.2), and to identify factors enabling established patients with rheumatoid arthritis (RA) to remain ADA-free.
Methods: Of 197 RA patients treated with ADA+methotrexate (MTX), 75 patients who met the ADA-free criteria (steroid-free and sustained DAS28-ESR remission for 6 months with stable MTX doses) were studied for 1 year.
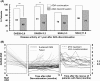
Results: The mean disease duration and DAS28-ESR score in 75 patients was 7.5 years and 5.1 at baseline, respectively. The proportion of patients who sustained DAS28-ESR <2.6 (48%) and DAS28-ESR <3.2 (62%) for 1 year were significantly lower in the ADA discontinuation group than in the ADA continuation group; however, in patients with deep remission (DAS28-ESR ≤1.98) identified by receiver operating characteristics analysis following logistic analysis, these rates increased to 68% and 79%, respectively, with no significant difference between both groups. Remarkably, ADA readministration to patients with flare was effective in returning DAS28-ESR to <3.2 within 6 months in 90% and 9 months in 100% patients; among the patients who sustained DAS28-ESR <3.2 during ADA discontinuation, 100% remained in structural remission and 94% in functional remission.
Conclusions: The possibility of remaining ADA-free for 1 year was demonstrated in established patients with RA with outcomes that ADA can be discontinued without flaring in 79% patients with deep remission, with similar rates in the ADA continuation group, and showed no functional or structural damage in patients with DAS28-ESR <3.2. ADA readministration to patients with flare during ADA discontinuation was effective.
Keywords: Anti-TNF; Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
-
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358:903–11. - PubMed
-
- Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol 2002;2:527–35. - PubMed
-
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001;344:907–16. - PubMed
-
- Clark DA. Do anti-TNF-α drugs increase cancer risk in rheumatoid arthritis patients? Inflammopharmacology 2013;21:125–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

