89Zr-Radiolabeled Trastuzumab Imaging in Orthotopic and Metastatic Breast Tumors
- PMID: 24288044
- PMCID: PMC3763623
- DOI: 10.3390/ph5010079
89Zr-Radiolabeled Trastuzumab Imaging in Orthotopic and Metastatic Breast Tumors
Abstract
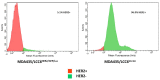
The human epidermal growth factor receptor 2 (HER2/neu) is overexpressed in 20-30% of breast cancers and is associated with tumor growth, angiogenesis, and development of distant metastases. Trastuzumab, an anti-HER2 monoclonal antibody, is used for the treatment of HER2 positive breast cancer and clinical efficacy of this agent is dependent on HER2 expression. Targeted PET imaging of HER2 with radiolabeled trastuzumab may be used to determine HER2 expression levels and guide therapy selection. The purpose of the current study was to evaluate a facile 89Zr-trastuzumab preparation method that can be efficiently applied for clinical grade production. Also, relative HER2 expression levels in orthotopic and metastatic breast cancer models were assessed by PET imaging using the 89Zr-trastuzumab produced by this simpler method.
Figures




References
-
- Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon D.J., Godolphin W., Jones L.A., Holt J.A., Wong S.G., Keith D.E., Levin W.J., Stuart S.G., Udove J., Ullrich A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

