Differential localization of NT-3 and TrpM5 in glomeruli of the olfactory bulb of mice
- PMID: 24288162
- PMCID: PMC3984597
- DOI: 10.1002/cne.23512
Differential localization of NT-3 and TrpM5 in glomeruli of the olfactory bulb of mice
Abstract
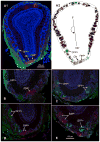
Olfactory sensory neurons that express transient receptor potential channel M5 (TrpM5) or neurotrophin-3 (NT-3) project to defined clusters of glomeruli situated ventrally in the main olfactory bulb. Using genetically labeled mice, we investigated whether expression of NT-3-driven βgal and TrpM5-driven GFP marked overlapping sets of glomeruli and whether expression of these markers was coordinated. Our results indicate that these markers largely characterize independent sets of olfactory sensory neuron axons and glomeruli. Further, in glomeruli in which both TrpM5-GFP and NT-3-βgal labeled axons occur, they are expressed independently. The nature of staining for these two markers also differs within glomeruli. Within each labeled TrpM5-positive glomerulus, the level of TrpM5-GFP expression was similar throughout the glomerular neuropil. In contrast, NT-3-driven βgal expression levels are heterogeneous even within heavily labeled glomeruli. In addition, a population of very small TrpM5-GFP positive glomeruli is apparent while no similar populations of NT-3-βgal glomeruli are evident. Taken together, these data suggest that TrpM5 and NT-3 characterize two largely independent receptor populations both conveying odorant information to the ventral olfactory bulb.
Keywords: olfaction, immunochemistry, mapping, semiochemical, microglomeruli.
Copyright © 2013 Wiley Periodicals, Inc.
Conflict of interest statement
Figures




Similar articles
-
Second-order input to the medial amygdala from olfactory sensory neurons expressing the transduction channel TRPM5.J Comp Neurol. 2012 Jun 1;520(8):1819-30. doi: 10.1002/cne.23015. J Comp Neurol. 2012. PMID: 22120520 Free PMC article.
-
Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals.Proc Natl Acad Sci U S A. 2007 Feb 13;104(7):2471-6. doi: 10.1073/pnas.0610201104. Epub 2007 Jan 31. Proc Natl Acad Sci U S A. 2007. PMID: 17267604 Free PMC article.
-
Chemical Exposure-Induced Changes in the Expression of Neurotrophins and Their Receptors in the Main Olfactory System of Mice Lacking TRPM5-Expressing Microvillous Cells.Int J Mol Sci. 2018 Sep 27;19(10):2939. doi: 10.3390/ijms19102939. Int J Mol Sci. 2018. PMID: 30261693 Free PMC article.
-
Grouping and representation of odorant receptors in domains of the olfactory bulb sensory map.Microsc Res Tech. 2002 Aug 1;58(3):168-75. doi: 10.1002/jemt.10146. Microsc Res Tech. 2002. PMID: 12203695 Review.
-
Grouping of odorant receptors: odour maps in the mammalian olfactory bulb.Biochem Soc Trans. 2003 Feb;31(Pt 1):134-6. doi: 10.1042/bst0310134. Biochem Soc Trans. 2003. PMID: 12546670 Review.
Cited by
-
Mammalian pheromones: emerging properties and mechanisms of detection.Curr Opin Neurobiol. 2015 Oct;34:103-9. doi: 10.1016/j.conb.2015.02.005. Epub 2015 Mar 6. Curr Opin Neurobiol. 2015. PMID: 25747731 Free PMC article. Review.
-
Nutrient Sensing: Another Chemosensitivity of the Olfactory System.Front Physiol. 2017 Jul 12;8:468. doi: 10.3389/fphys.2017.00468. eCollection 2017. Front Physiol. 2017. PMID: 28747887 Free PMC article. Review.
-
Linear correlation between the number of olfactory sensory neurons expressing a given mouse odorant receptor gene and the total volume of the corresponding glomeruli in the olfactory bulb.J Comp Neurol. 2016 Jan 1;524(1):199-209. doi: 10.1002/cne.23835. Epub 2015 Jul 16. J Comp Neurol. 2016. PMID: 26100963 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

