Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI
- PMID: 24288260
- PMCID: PMC4019439
- DOI: 10.1002/nbm.3048
Assessment of ischemic penumbra in patients with hyperacute stroke using amide proton transfer (APT) chemical exchange saturation transfer (CEST) MRI
Abstract
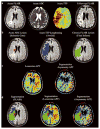
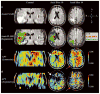
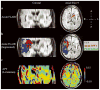
Chemical exchange saturation transfer (CEST)-derived, pH-weighted, amide proton transfer (APT) MRI has shown promise in animal studies for the prediction of infarction risk in ischemic tissue. Here, APT MRI was translated to patients with acute stroke (1-24 h post-symptom onset), and assessments of APT contrast, perfusion, diffusion, disability and final infarct volume (23-92 days post-stroke) are reported. Healthy volunteers (n = 5) and patients (n = 10) with acute onset of symptoms (0-4 h, n = 7; uncertain onset <24 h, n = 3) were scanned with diffusion- and perfusion-weighted MRI, fluid-attenuated inversion recovery (FLAIR) and CEST. Traditional asymmetry and a Lorentzian-based APT index were calculated in the infarct core, at-risk tissue (time-to-peak, TTP; lengthening) and final infarct volume. On average (mean ± standard deviation), control white matter APT values (asymmetry, 0.019 ± 0.005; Lorentzian, 0.045 ± 0.006) were not significantly different (p > 0.05) from APT values in normal-appearing white matter (NAWM) of patients (asymmetry, 0.022 ± 0.003; Lorentzian, 0.048 ± 0.003); however, ischemic regions in patients showed reduced (p = 0.03) APT effects compared with NAWM. Representative cases are presented, whereby the APT contrast is compared quantitatively with contrast from other imaging modalities. The findings vary between patients; in some patients, a trend for a reduction in the APT signal in the final infarct region compared with at-risk tissue was observed, consistent with tissue acidosis. However, in other patients, no relationship was observed in the infarct core and final infarct volume. Larger clinical studies, in combination with focused efforts on sequence development at clinically available field strengths (e.g. 3.0 T), are necessary to fully understand the potential of APT imaging for guiding the hyperacute management of patients.
Keywords: MRI; acute stroke; amide proton transfer; cerebrovascular disease; chemical exchange saturation transfer (CEST); lactate; pH; penumbra.
Copyright © 2013 John Wiley & Sons, Ltd.
Conflict of interest statement
The authors do not have any conflict of interest to disclose with regards to the material in this manuscript.
Figures





References
-
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischemic stroke. The New England journal of medicine. 2008;359(13):1317–1329. - PubMed
-
- Wechsler LR. Intravenous thrombolytic therapy for acute ischemic stroke. The New England journal of medicine. 2011;364(22):2138–2146. - PubMed
-
- Hesselmann V, Niederstadt T, Dziewas R, Ritter M, Kemmling A, Maintz D, Koehler M, Seifarth H, Jacobs AH, Ringelstein EB, Heindel W. Reperfusion by combined thrombolysis and mechanical thrombectomy in acute stroke: effect of collateralization, mismatch, and time to and grade of recanalization on clinical and tissue outcome. AJNR Am J Neuroradiol. 2012;33(2):336–342. - PMC - PubMed
-
- Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, von Kummer R, Molina CA, Demaerschalk BM, Budzik R, Clark WM, Zaidat OO, Malisch TW, Goyal M, Schonewille WJ, Mazighi M, Engelter ST, Anderson C, Spilker J, Carrozzella J, Ryckborst KJ, Janis LS, Martin RH, Foster LD, Tomsick TA. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. The New England journal of medicine. 2013;368(10):893–903. - PMC - PubMed
-
- Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, Zeumer H, Rother J. Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke. 2002;33(1):79–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

