Management of adenovirus in children after allogeneic hematopoietic stem cell transplantation
- PMID: 24288536
- PMCID: PMC3830830
- DOI: 10.1155/2013/176418
Management of adenovirus in children after allogeneic hematopoietic stem cell transplantation
Abstract
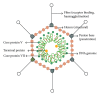
Adenovirus (ADV) can cause significant morbidity and mortality in children following haematopoietic stem cell transplantation (HSCT), with an incidence of up to 27% and notable associated morbidity and mortality. T-cell depleted grafts and severe lymphopenia are major risk factors for the development of adenovirus disease after HSCT. Current antiviral treatments are at best virostatic and may have significant side effects. Adoptive transfer of donor-derived virus-specific T cells has been shown to be an effective strategy for the prevention and treatment of ADV infection after HSCT. Here we review progress in the field and present a pathway for the management of adenovirus in the posttransplant setting.
Figures
Similar articles
-
Curative or pre-emptive adenovirus-specific T cell transfer from matched unrelated or third party haploidentical donors after HSCT, including UCB transplantations: a successful phase I/II multicenter clinical trial.J Hematol Oncol. 2017 May 8;10(1):102. doi: 10.1186/s13045-017-0469-0. J Hematol Oncol. 2017. PMID: 28482908 Free PMC article. Clinical Trial.
-
Occurrence, risk factors and outcome of adenovirus infection in adult recipients of allogeneic hematopoietic stem cell transplantation.J Clin Virol. 2016 Sep;82:33-40. doi: 10.1016/j.jcv.2016.07.002. Epub 2016 Jul 10. J Clin Virol. 2016. PMID: 27428881
-
Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival.Biol Blood Marrow Transplant. 2003 May;9(5):341-52. doi: 10.1016/s1083-8791(03)00102-2. Biol Blood Marrow Transplant. 2003. PMID: 12766884
-
Strategies of adoptive T -cell transfer to treat refractory viral infections post allogeneic stem cell transplantation.J Hematol Oncol. 2019 Feb 6;12(1):13. doi: 10.1186/s13045-019-0701-1. J Hematol Oncol. 2019. PMID: 30728058 Free PMC article. Review.
-
Adenovirus infection after allogeneic stem cell transplantation.Leuk Lymphoma. 2007 Feb;48(2):244-55. doi: 10.1080/10428190600881157. Leuk Lymphoma. 2007. PMID: 17325884 Review.
Cited by
-
CD46-mediated transduction of a species D adenovirus vaccine improves mucosal vaccine efficacy.Hum Gene Ther. 2014 Apr;25(4):364-74. doi: 10.1089/hum.2013.215. Epub 2014 Apr 10. Hum Gene Ther. 2014. PMID: 24635714 Free PMC article.
-
Viral-specific T-cell transfer from HSCT donor for the treatment of viral infections or diseases after HSCT.Bone Marrow Transplant. 2018 Feb;53(2):114-122. doi: 10.1038/bmt.2017.232. Epub 2017 Oct 23. Bone Marrow Transplant. 2018. PMID: 29058697 Review.
-
Scheduled administration of virus-specific T cells for viral prophylaxis after pediatric allogeneic stem cell transplant.Blood Adv. 2022 May 10;6(9):2897-2907. doi: 10.1182/bloodadvances.2021006309. Blood Adv. 2022. PMID: 35108727 Free PMC article. Clinical Trial.
-
Acute Neurological Involvement after Donor Lymphocyte Infusion for Post-Transplant Viral Infection: The Same Pattern of Novel Cancer Immunotherapy-Related CNS Toxicity?Int J Mol Sci. 2022 Mar 24;23(7):3553. doi: 10.3390/ijms23073553. Int J Mol Sci. 2022. PMID: 35408912 Free PMC article.
-
Identification of a novel intertypic recombinant species D human adenovirus in a pediatric stem cell transplant recipient.J Clin Virol. 2014 Dec;61(4):496-502. doi: 10.1016/j.jcv.2014.09.009. Epub 2014 Sep 28. J Clin Virol. 2014. PMID: 25449172 Free PMC article.
References
-
- Rowe WP, Huebner RJ, Gilmore LK, et al. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proceedings of the Society for Experimental Biology and Medicine. 1953;84(3):570–573. - PubMed
-
- Walls T, Shankar AG, Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. The Lancet Infectious Diseases. 2003;3(2):79–86. - PubMed
-
- Heim A. Advances in the management of disseminated adenovirus disease in stem cell transplant recipients: impact of adenovirus load (DNAemia) testing. Expert Review of Anti-Infective Therapy. 2011;9(11):943–945. - PubMed
-
- Lion T, Baumgartinger R, Watzinger F, et al. Molecular monitoring of adenovirus in peripheral blood after allogeneic bone marrow transplantation permits early diagnosis of disseminated disease. Blood. 2003;102(3):1114–1120. - PubMed
-
- Kojaoghlanian T, Flomenberg P, Horwitz MS. The impact of adenovirus infection on the immunocompromised host. Reviews in Medical Virology. 2003;13(3):155–171. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

