Protocol for the New Medicine Service Study: a randomized controlled trial and economic evaluation with qualitative appraisal comparing the effectiveness and cost effectiveness of the New Medicine Service in community pharmacies in England
- PMID: 24289059
- PMCID: PMC4220816
- DOI: 10.1186/1745-6215-14-411
Protocol for the New Medicine Service Study: a randomized controlled trial and economic evaluation with qualitative appraisal comparing the effectiveness and cost effectiveness of the New Medicine Service in community pharmacies in England
Abstract
Background: Medication non-adherence is considered an important cause of morbidity and mortality in primary care. This study aims to determine the effectiveness, cost effectiveness and acceptability of a complex intervention delivered by community pharmacists, the New Medicine Service (NMS), compared with current practice in reducing non-adherence to, and problems with, newly prescribed medicines for chronic conditions.
Methods/design: Research subject group: patients aged 14 years and above presenting in a community pharmacy for a newly prescribed medicine for asthma/chronic obstructive pulmonary disease (COPD); hypertension; type 2 diabetes or anticoagulant/antiplatelet agents in two geographical regions in England.
Design: parallel group patient-level pragmatic randomized controlled trial.
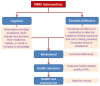
Interventions: patients randomized to either: (i) current practice; or (ii) NMS intervention comprising pharmacist-delivered support for a newly prescribed medicine.
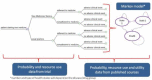
Primary outcomes: proportion of adherent patients at six, ten and 26 weeks from the date of presenting their prescriptions at the pharmacy; cost effectiveness of the intervention versus current practice at 10 weeks and 26 weeks; in-depth qualitative understanding of the operationalization of NMS in pharmacies.
Secondary outcomes: impact of NMS on: patients' understanding of their medicines, pharmacovigilance, interprofessional and patient-professional relationships and experiences of service users and stakeholders.Economic analysis: Trial-based economic analysis (cost per extra adherent patient) and long-term modeling of costs and health effects (cost per quality-adjusted-life-year) will be conducted from the perspective of National Health Service (NHS) England, comparing NMS with current practice.Qualitative analysis: a qualitative study of NMS implementation in different community settings, how organizational influences affect NMS delivery, patterns of NMS consultations and experiences of professionals and patients participating in NMS, and patients receiving current practice.
Sample size: 250 patients in each treatment arm would provide at least 80% power (two-tailed alpha of 0.05) to demonstrate a reduction in patient-reported non-adherence from 20% to 10% in the NMS arm compared with current practice, assuming a 20% drop-out rate.
Discussion: At the time of submission of this article, 58 community pharmacies have been recruited and the interventions are being delivered. Analysis has not yet been undertaken.
Trial registration: Current controlled trials: ISRCTN23560818. Clinical Trials US (clinicaltrials.gov): NCT01635361.
Figures
References
-
- World Health Organization. Adherence to Long-term Therapies. Evidence for Action. 2003. http://whqlibdoc.who.int/publications/2003/9241545992.pdf.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical