GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer
- PMID: 24289103
- PMCID: PMC3978564
- DOI: 10.1186/bcr3581
GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer
Abstract
Introduction: Tamoxifen is widely used to treat hormone-dependent breast cancer, but its therapeutic benefit is limited by the development of drug resistance. Here, we investigated the role of estrogen G-protein coupled receptor 30 (GPR30) on Tamoxifen resistance in breast cancer.
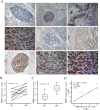
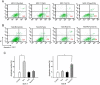
Methods: Primary tumors (PTs) of breast cancer and corresponding metastases (MTs) were used to evaluate the expression of GPR30 and epidermal growth factor receptor (EGFR) immunohistochemically. Tamoxifen-resistant (TAM-R) subclones derived from parent MCF-7 cells were used to investigate the role of GPR30 in the development of tamoxifen resistance, using MTT assay, western blot, RT-PCR, immunofluorescence, ELISA and flow cytometry. TAM-R xenografts were established to assess anti-tumor effects of combination therapy with GPR30 antagonist G15 plus 4-hydroxytamoxifen (Tam), using tumor volume measurement and Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).
Results: In 53 human breast cancer specimens, GPR30 expression in MTs increased compared to matched PTs; in MTs, the expression patterns of GPR30 and EGFR were closely related. Compared to parent MCF-7 cells, TAM-R cells had greater growth responses to 17β-estradiol (E2), GPR30 agonist G1 and Tam, and significantly higher activation of Mitogen-activated protein (MAP) kinases; but this increased activity was abolished by G15 or AG1478. In TAM-R cells, GPR30 cell-surface translocation facilitated crosstalk with EGFR, and reduced cAMP generation, attenuating inhibition of EGFR signaling. Combination therapy both promoted apoptosis in TAM-R cells and decreased drug-resistant tumor progression.
Conclusions: Long-term endocrine treatment facilitates the translocation of GPR30 to cell surfaces, which interferes with the EGFR signaling pathway; GPR30 also attenuates the inhibition of MAP kinases. These factors contribute to tamoxifen resistance development in breast cancer. Combination therapy with GPR30 inhibitors and tamoxifen may provide a new therapeutic option for drug-resistant breast cancer.
Figures
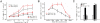



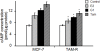

Similar articles
-
Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells.Breast Cancer Res Treat. 2010 Aug;123(1):87-96. doi: 10.1007/s10549-009-0624-6. Epub 2009 Nov 13. Breast Cancer Res Treat. 2010. PMID: 19911269
-
GPR30-mediated HMGB1 upregulation in CAFs induces autophagy and tamoxifen resistance in ERα-positive breast cancer cells.Aging (Albany NY). 2021 Jun 28;13(12):16178-16197. doi: 10.18632/aging.203145. Epub 2021 Jun 28. Aging (Albany NY). 2021. PMID: 34182538 Free PMC article.
-
Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: a new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and β1-integrin signaling pathway in tumor cells.Breast Cancer Res. 2015 May 21;17(1):69. doi: 10.1186/s13058-015-0579-y. Breast Cancer Res. 2015. PMID: 25990368 Free PMC article.
-
Tamoxifen Resistance: Emerging Molecular Targets.Int J Mol Sci. 2016 Aug 19;17(8):1357. doi: 10.3390/ijms17081357. Int J Mol Sci. 2016. PMID: 27548161 Free PMC article. Review.
-
Mechanisms of tamoxifen resistance in the treatment of advanced breast cancer.Acta Oncol. 1996;35 Suppl 5:9-14. doi: 10.3109/02841869609083961. Acta Oncol. 1996. PMID: 9142958 Review.
Cited by
-
The insulin-like growth factor-I receptor (IGF-IR) in breast cancer: biology and treatment strategies.Tumour Biol. 2016 Sep;37(9):11711-11721. doi: 10.1007/s13277-016-5176-x. Epub 2016 Jul 21. Tumour Biol. 2016. PMID: 27444280 Review.
-
Moonlighting Proteins and Cardiopathy in the Spatial Response of MCF-7 Breast Cancer Cells to Tamoxifen.Proteomics Clin Appl. 2019 Sep;13(5):e1900029. doi: 10.1002/prca.201900029. Epub 2019 Jul 25. Proteomics Clin Appl. 2019. PMID: 31282103 Free PMC article.
-
IGF-1 Receptor Modulates FoxO1-Mediated Tamoxifen Response in Breast Cancer Cells.Mol Cancer Res. 2017 Apr;15(4):489-497. doi: 10.1158/1541-7786.MCR-16-0176. Epub 2017 Jan 17. Mol Cancer Res. 2017. PMID: 28096479 Free PMC article.
-
Estrogen Activation by Steroid Sulfatase Increases Colorectal Cancer Proliferation via GPER.J Clin Endocrinol Metab. 2017 Dec 1;102(12):4435-4447. doi: 10.1210/jc.2016-3716. J Clin Endocrinol Metab. 2017. PMID: 28945888 Free PMC article.
-
The Interplay of GPER1 with 17β-Aminoestrogens in the Regulation of the Proliferation of Cervical and Breast Cancer Cells: A Pharmacological Approach.Int J Environ Res Public Health. 2022 Sep 28;19(19):12361. doi: 10.3390/ijerph191912361. Int J Environ Res Public Health. 2022. PMID: 36231664 Free PMC article.
References
-
- Briest S, Stearns V. Tamoxifen metabolism and its effect on endocrine treatment of breast cancer. Clin Adv Hematol Oncol. 2009;15:185–192. - PubMed
-
- Delozier T, Spielmann M, Mace-Lesec'h J, Janvier M, Hill C, Asselain B, Julien JP, Weber B, Mauriac L, Petit JC, Kerbrat P, Malhaire JP, Vennin P, Leduc B, Namer M. Tamoxifen adjuvant treatment duration in early breast cancer: initial results of a randomized study comparing short-term treatment with long-term treatment, Federation Nationale des Centres de Lutte Contre le Cancer Breast Group. J Clin Oncol. 2000;15:3507–3512. - PubMed
-
- van Agthoven T, Sieuwerts AM, Meijer-van Gelder ME, Look MP, Smid M, Veldscholte J, Sleijfer S, Foekens JA, Dorssers LC. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J Clin Oncol. 2009;15:542–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

