Reticulocyte-prone malaria parasites predominantly invade CD71hi immature cells: implications for the development of an in vitro culture for Plasmodium vivax
- PMID: 24289105
- PMCID: PMC4220676
- DOI: 10.1186/1475-2875-12-434
Reticulocyte-prone malaria parasites predominantly invade CD71hi immature cells: implications for the development of an in vitro culture for Plasmodium vivax
Abstract
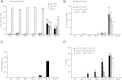
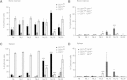
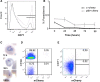
Background: The lack of a continuous in vitro culture system for blood stages of malarial parasites with a unique tropism for reticulocytes, such as Plasmodium vivax and the Plasmodium yoelii 17X reticulocyte-prone strain, hinders research in these organisms. The maturation of reticulocytes into erythrocytes is a complex process involving the selective removal of membrane proteins such as the transferrin receptor, CD71. In order to advance in the characterization of infected cells during experimental infections of BALB/c mice with P. yoelii 17X, CD71 expression in erythroid cells (TER119+) was assessed and in vitro culture of P. yoelii 17X was attempted by adding reticulocytes highly expressing CD71.
Methods: BALB/c mice were infected with P. yoelii 17X-GFP transgenic parasites and erythroid cells (TER119+) were analysed in blood, spleen and bone marrow cells. TER119, CD71 and GFP expression was assessed at different points post-infection by flow cytometry. Moreover, in vitro culture of P. yoelli 17X was attempted by adding red blood cells (RBCs) from mice with a pyruvate kinase deficiency, which contain high percentages of CD71hi cells in peripheral blood as compared to healthy animals.
Results: A predominance of erythroid cells lacking expression of CD71 (CD71-) was observed in peripheral blood and spleen in normal and infected animals up to ten days post-infection (pi). At ten days pi, however, a dramatic temporal switch to erythroid cells highly expressing CD71 (CD71hi) was observed in the spleen and at day 15 pi in peripheral blood of the infected cells. A distribution of erythroid cells expressing differently CD71 was noticed in the bone marrow. Yet, similar to peripheral blood and spleen, a predominance of CD71hi cells was observed at 15 days pi. Remarkably, CD71hi cells were the cells predominantly infected in these organs as well as in peripheral blood. Attempts were thus made to culture in vitro the P. yoelli 17X strain by adding RBCs from pyruvate kinase-deficient mice containing high percentages of CD71hi cells in peripheral blood.
Conclusions: The parasite preference for immature cells that are rare in normal peripheral blood could have important implications for the development of an in vitro culture system for P. vivax.
Figures


Similar articles
-
Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes.Blood. 2015 Feb 19;125(8):1314-24. doi: 10.1182/blood-2014-08-596015. Epub 2014 Nov 20. Blood. 2015. PMID: 25414440 Free PMC article.
-
Unambiguous determination of Plasmodium vivax reticulocyte invasion by flow cytometry.Int J Parasitol. 2016 Jan;46(1):31-9. doi: 10.1016/j.ijpara.2015.08.003. Epub 2015 Sep 15. Int J Parasitol. 2016. PMID: 26385436
-
Targeting anemia-induced CD71+ reticulocytes protects mice from Plasmodium infection.Infect Immun. 2025 Aug 12;93(8):e0009325. doi: 10.1128/iai.00093-25. Epub 2025 Jul 1. Infect Immun. 2025. PMID: 40590713 Free PMC article.
-
The unhealthy attraction of Plasmodium vivax to reticulocytes expressing transferrin receptor 1 (CD71).Int J Parasitol. 2017 Jun;47(7):379-383. doi: 10.1016/j.ijpara.2017.03.001. Epub 2017 Apr 13. Int J Parasitol. 2017. PMID: 28414070 Review.
-
Molecular and cellular interactions defining the tropism of Plasmodium vivax for reticulocytes.Curr Opin Microbiol. 2018 Dec;46:109-115. doi: 10.1016/j.mib.2018.10.002. Epub 2018 Oct 23. Curr Opin Microbiol. 2018. PMID: 30366310 Free PMC article. Review.
Cited by
-
Contributions of IFN-γ and granulysin to the clearance of Plasmodium yoelii blood stage.PLoS Pathog. 2020 Sep 10;16(9):e1008840. doi: 10.1371/journal.ppat.1008840. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32913355 Free PMC article.
-
The Epimmunity Theory: The Single Cell Defenses against Infectious and Genetic Diseases.Front Immunol. 2017 Jun 13;8:694. doi: 10.3389/fimmu.2017.00694. eCollection 2017. Front Immunol. 2017. PMID: 28659926 Free PMC article.
-
Immature reticulocytes as preferential host cells and the challenges for in vitro culture of Plasmodium vivax.Pathog Glob Health. 2015 May;109(3):91-2. doi: 10.1179/2047772415Z.000000000264. Pathog Glob Health. 2015. PMID: 25943155 Free PMC article. No abstract available.
-
Severe malaria enforces short-lived effector cell differentiation but does not prevent effective secondary responses by memory CD8 T cells.PLoS Pathog. 2025 Mar 31;21(3):e1012993. doi: 10.1371/journal.ppat.1012993. eCollection 2025 Mar. PLoS Pathog. 2025. PMID: 40163479 Free PMC article.
-
Malaria modeling: In vitro stem cells vs in vivo models.World J Stem Cells. 2016 Mar 26;8(3):88-100. doi: 10.4252/wjsc.v8.i3.88. World J Stem Cells. 2016. PMID: 27022439 Free PMC article. Review.
References
-
- Kitchen SK. The infection of reticulocytes by Plasmodium vivax. Am J Trop Med Hyg. 1938;18:347.
-
- Bessis M. [Ultrastructure of the Cells of the Erythrocytic Series](in Italian) Arch Ital Anat Istol Patol. 1963;37:541–549. - PubMed
-
- Heilmeyer LaW R. Reifungs-studien an überlebenden Recticulocyten in vitro and ihre Bedeutung für die Schätzing der täglichen Haemoglobin-produktion in vivo. Z Klin Med. 1932;121:361–379.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

